Table of Contents
Introduction
Methotrexate is a cornerstone drug in both anticancer chemotherapy and immunosuppressive therapy. Structurally, it is a folic acid antagonist and functions as an antimetabolite.
It is widely used in:
- Various malignancies like acute lymphoblastic leukemia (ALL)
- Autoimmune diseases such as rheumatoid arthritis and psoriasis
- Ectopic pregnancy management
Due to its dual role in oncology and immunology, Methotrexate is a high-yield topic in exams like GPAT, NIPER, NEET-PG, NCLEX, and essential for PharmD, MBBS, and B.Pharm students.
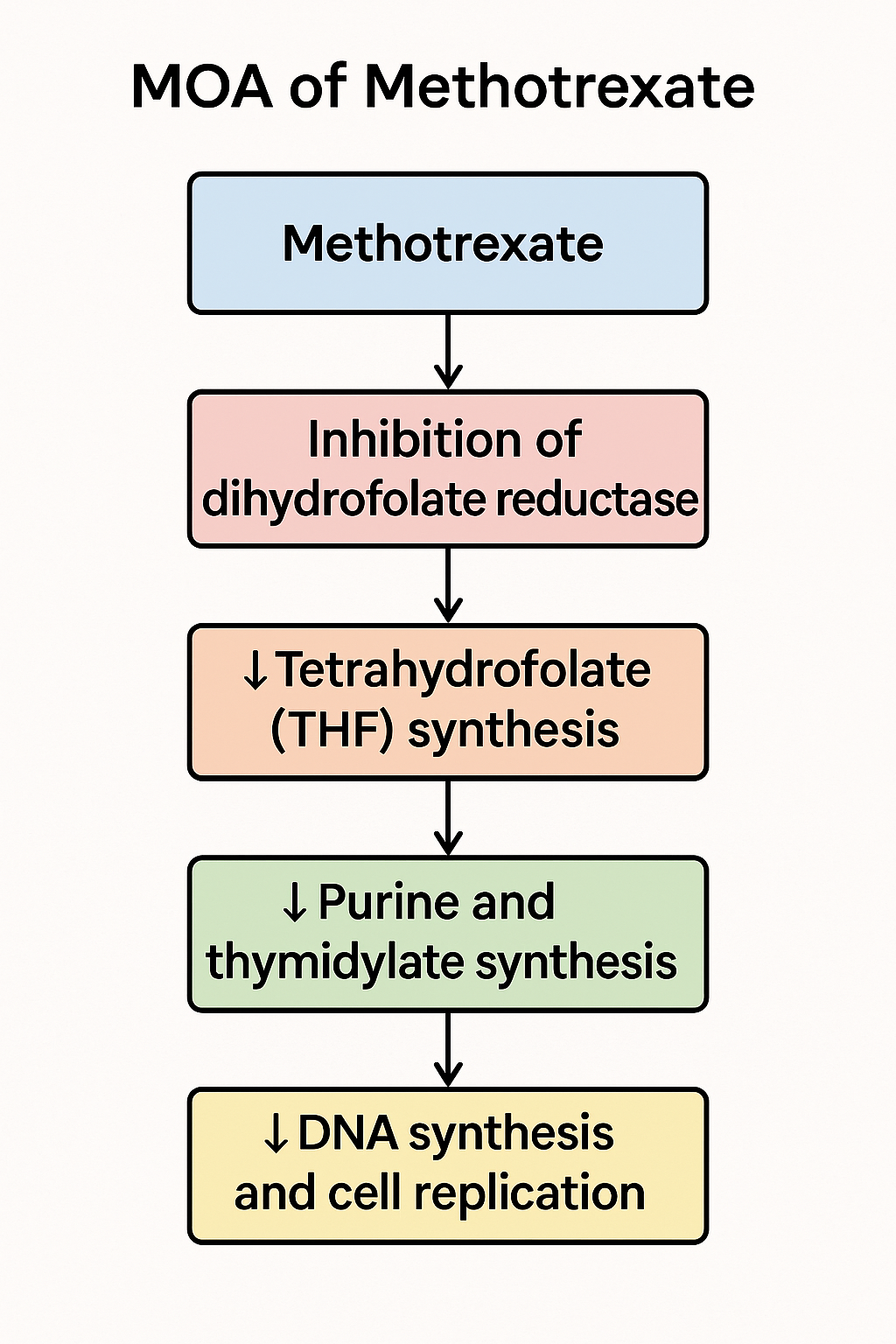
Stepwise Mechanism of Action of Methotrexate
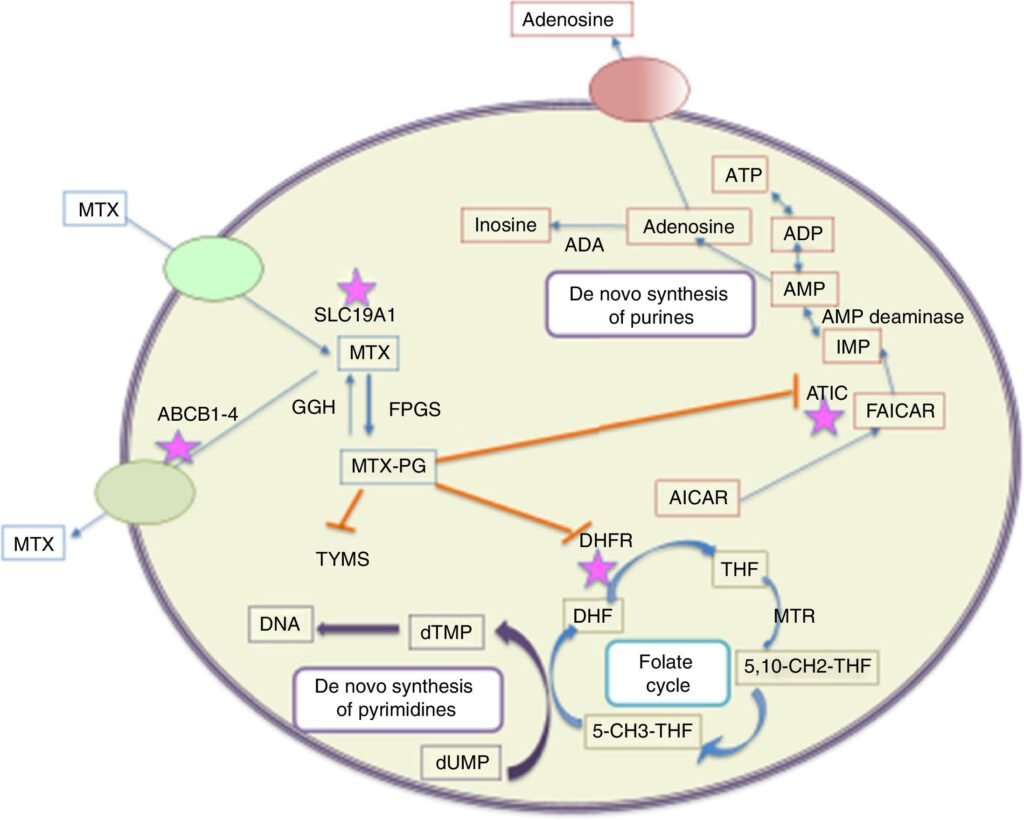
- Cellular Uptake:
Methotrexate enters the cell via the reduced folate carrier (RFC), mimicking folic acid. - Inhibition of Dihydrofolate Reductase (DHFR):
Methotrexate competitively inhibits DHFR, the enzyme that converts dihydrofolate (DHF) to tetrahydrofolate (THF). THF is essential for purine and thymidylate (dTMP) synthesis. - ↓ THF → Impaired DNA Synthesis:
Lack of THF disrupts one-carbon transfer reactions needed for purine (adenine, guanine) and thymidylate synthesis, leading to defective DNA replication. - Cell Cycle Arrest in S-phase:
Rapidly dividing cells are most affected, especially in the S-phase, where DNA synthesis is active. This leads to cytotoxicity in malignant cells and immunosuppression in autoimmune diseases. - Polyglutamation & Prolonged Action:
Inside the cell, methotrexate undergoes polyglutamation, which enhances retention and sustains DHFR inhibition, making its effects more long-lasting. - Additional Anti-inflammatory Mechanism:
Methotrexate also promotes adenosine release, which contributes to its anti-inflammatory action in diseases like rheumatoid arthritis.
Pharmacokinetic Parameters of Methotrexate
| Parameter | Value |
|---|---|
| Bioavailability | 70–90% (oral, low dose); lower at high doses |
| Onset of action | Weeks (in autoimmune diseases) |
| Half-life | 3–10 hours (low dose), up to 15 hours (high dose) |
| Protein binding | ~50% |
| Metabolism | Liver (partial), polyglutamation in tissues |
| Excretion | Primarily renal (glomerular filtration & secretion) |
Clinical Uses of Methotrexate
Antineoplastic Indications:
- Acute lymphoblastic leukemia (ALL)
- Choriocarcinoma
- Non-Hodgkin’s lymphoma
- Breast and head & neck cancers
Immunosuppressive/Anti-inflammatory Uses:
- Rheumatoid arthritis (low-dose, weekly)
- Psoriasis
- Systemic lupus erythematosus (off-label)
- Ectopic pregnancy
Adverse Effects of Methotrexate
- Myelosuppression (neutropenia, anemia, thrombocytopenia)
- Hepatotoxicity (elevated transaminases, fibrosis)
- Pulmonary fibrosis and pneumonitis
- Stomatitis and mucositis
- Teratogenicity (Category X in pregnancy)
- GI upset, nausea, diarrhea
Folinic acid (Leucovorin) rescue is used to prevent toxicity in high-dose therapy.
Comparative Analysis: Methotrexate vs Azathioprine
| Feature | Methotrexate | Azathioprine |
|---|---|---|
| Class | Antimetabolite (Folate antagonist) | Purine analog |
| Primary Action | Inhibits DHFR → ↓ DNA synthesis | Inhibits purine synthesis (via 6-MP) |
| Use in RA | First-line DMARD | Second-line agent |
| Onset of Action | 4–6 weeks | 6–12 weeks |
| Monitoring | CBC, LFTs, renal | CBC, LFTs |
| Common Side Effects | Myelosuppression, hepatotoxicity, lung tox. | Myelosuppression, hepatotoxicity |
Practice MCQs: Methotrexate
Q1. Methotrexate belongs to which class of drugs?
A. Alkylating agent
B. Folic acid antagonist ✅
C. Purine analog
D. Topoisomerase inhibitor
Q2. The primary site of action of methotrexate is:
A. Thymidylate synthase
B. Dihydrofolate reductase ✅
C. Adenylosuccinate synthetase
D. DNA polymerase
Q3. Which of the following pathways is directly blocked by methotrexate?
A. Pyrimidine methylation
B. One-carbon folate pathway ✅
C. GABA synthesis
D. Cholesterol biosynthesis
Q4. What is the main result of methotrexate-induced inhibition of tetrahydrofolate synthesis?
A. Enhanced RNA polymerase activity
B. DNA synthesis inhibition ✅
C. Increased histamine release
D. Beta-lactamase activation
Q5. Which of the following drugs is used as a rescue agent to prevent methotrexate toxicity?
A. Folic acid
B. Vitamin B12
C. Leucovorin (Folinic acid) ✅
D. Prednisone
Q6. Methotrexate is most effective in which phase of the cell cycle?
A. G1
B. S ✅
C. G2
D. M
Q7. Which of the following is NOT an approved use of methotrexate?
A. Rheumatoid arthritis
B. Acute lymphoblastic leukemia
C. Psoriasis
D. Hypertension ✅
Q8. Which toxicity is most closely associated with long-term low-dose methotrexate?
A. Retinal damage
B. Hepatotoxicity ✅
C. Pancreatitis
D. Cardiotoxicity
Q9. Which statement about methotrexate excretion is true?
A. Excreted through bile
B. Eliminated via sweat
C. Excreted unchanged primarily through the kidneys ✅
D. Exhaled via lungs
Q10. Methotrexate is absolutely contraindicated in which condition?
A. Osteoarthritis
B. Pregnancy ✅
C. Male infertility
D. Anemia of chronic disease
FAQs
Q1: Can methotrexate be used in pregnancy?
No. It is absolutely contraindicated due to its teratogenic effects (FDA Category X).
Q2: What is the role of folinic acid (leucovorin)?
Folinic acid rescues normal cells by bypassing the DHFR blockade and preventing methotrexate toxicity.
Q3: Is methotrexate used in low or high doses for rheumatoid arthritis?
Low-dose, once-weekly methotrexate is the first-line DMARD for RA.
Q4: What monitoring is required during methotrexate therapy?
Regular CBC, liver function tests (LFTs), and renal function tests are essential to detect toxicity early.
References
- KD Tripathi – Essentials of Medical Pharmacology, 8th Edition
- Sparsh Gupta – Review of Pharmacology
- Goodman & Gilman – The Pharmacological Basis of Therapeutics

I am pursuing MBA in pharmaceutical management from NIPER Hyderabad with a strong academic record and proven success in national-level pharmacy entrance exams. I secured AIR 61 in NIPER 2024 (MS/M.Pharm) and AIR 27 in NIPER MBA, along with AIR 147 in GPAT 2024 and AIR 907 in GPAT 2023. I also achieved AIR 6 in AIIMS CRE-2025 for Drug Store Keeper and was selected as a Pharmacist (AIR 61) for ESIC. Additionally, I was the Runner-Up in Round 2 of the EY Case Study Competition.
At PharmacyFreak.com, I aim to guide future pharmacists through expert content, exam strategies, and insightful resources based on real experience and academic excellence.
Mail- harsh@pharmacyfreak.com