Table of Contents
1.Identification
Summary
First-line oral antihyperglycemic (biguanide) used in type 2 diabetes mellitus; lowers hepatic glucose output, improves insulin sensitivity, and has minimal risk of hypoglycemia when used alone.
Brand Names
Glucophage, Riomet, Fortamet, Glumetza (regional availability varies)
Name
Metformin
Background
A synthetic dimethylbiguanide introduced clinically in the mid-20th century; retained on essential-medicine lists worldwide due to efficacy, safety profile, and low cost. Available as immediate-release and extended-release oral formulations and in many fixed-dose combinations.
Modality
Small molecule
Groups
Approved; prescription (OTC status varies by jurisdiction)
Structure
Weight
~129.16 g/mol (base)
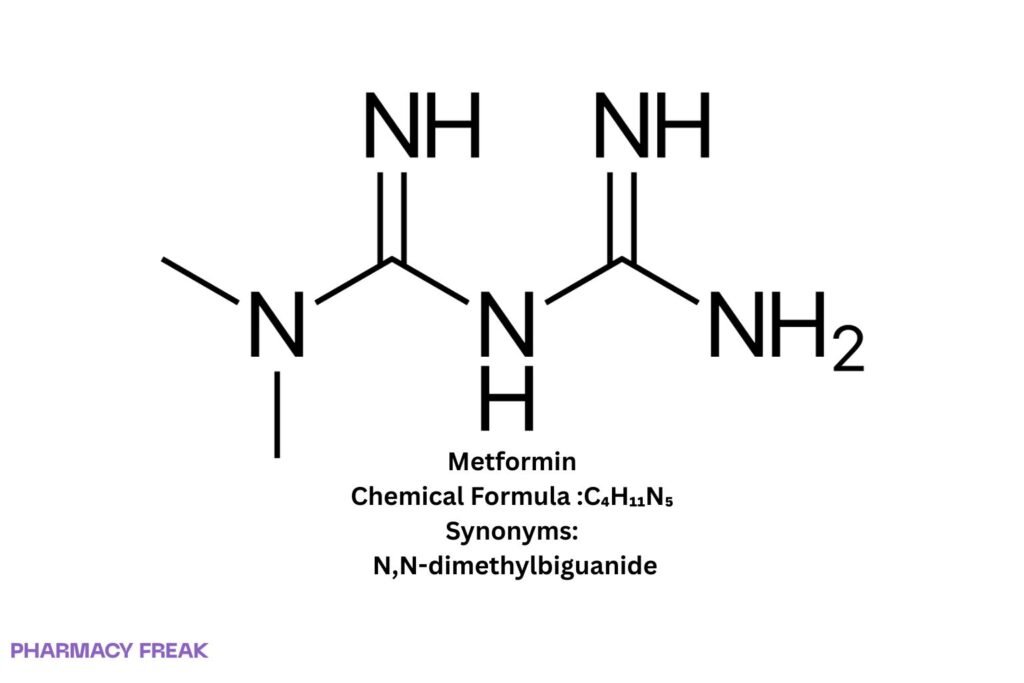
Chemical Formula
C₄H₁₁N₅
Synonyms
N,N-dimethylbiguanide
External IDs
CAS (base): 657-24-9; UNII (base): 9100L32L2N; PubChem CID: 4091; ChEMBL: CHEMBL1431; ChEBI: CHEBI:6801; DrugBank: DB00331
(Common salt) Metformin hydrochloride — CAS 1115-70-4; UNII 786Z46389E; PubChem CID 14219
2. Pharmacology
Indication
Adjunct to diet and exercise to improve glycemic control in adults and children with type 2 diabetes mellitus; widely used in combination with other antihyperglycemics. Off-label uses include PCOS and weight-neutral glucose control in insulin-resistant states (per local guidance).
Associated Conditions
Type 2 diabetes; insulin resistance; metabolic syndrome features; polycystic ovary syndrome (off-label)
Associated Therapies
Fixed-dose combinations with DPP-4 inhibitors, SGLT2 inhibitors, thiazolidinediones, sulfonylureas, or insulin (region-specific)
Contraindications & Blackbox Warnings
Boxed warning for lactic acidosis risk with drug accumulation; avoid or adjust in severe renal impairment and with acute conditions predisposing to hypoxia or dehydration. Review contrast-imaging precautions and alcohol use.
Pharmacodynamics
Antihyperglycemic (not hypoglycemic) at usual doses; reduces fasting plasma glucose and HbA1c primarily by suppressing hepatic gluconeogenesis, with complementary effects on intestinal glucose handling and peripheral insulin sensitivity.
Mechanism of action
Multiple converging mechanisms: inhibition of hepatic mitochondrial respiratory chain (Complex I) leading to AMPK activation; decreased gluconeogenic flux; increased GLUT4-mediated uptake in muscle; and gut-mediated effects (GLP-1 signaling, microbiome modulation).
Absorption
Oral bioavailability roughly 50–60% (fasted); IR tₘₐₓ ~1–3 h, ER tₘₐₓ ~4–8 h. Food delays absorption and can reduce peak exposure; ER forms improve GI tolerability.
Volume of distribution
Very large apparent Vd (hundreds of liters in adults), consistent with extensive tissue uptake via OCT transporters.
Protein binding
Negligible
Metabolism
Minimal hepatic metabolism; drug largely unchanged systemically.
Route of elimination
Renal excretion (active tubular secretion) of unchanged drug; clearance depends on kidney function.
Half-life
Plasma ~6 hours (longer in blood due to RBC compartment); prolonged in renal impairment/overdose.
Clearance
High renal clearance for the base; dose adjustments guided by eGFR thresholds per labeling.
Adverse Effects
Common: GI upset (nausea, diarrhea, abdominal discomfort), metallic taste—often mitigated by food or ER formulations. Rare: vitamin B₁₂ reduction with long-term high-dose use; lactic acidosis in predisposed patients.
Toxicity
Overdose or accumulation can precipitate metformin-associated lactic acidosis (MALA); management includes supportive care and, when indicated, hemodialysis.
Pathways
Transporter-mediated uptake (e.g., OCT1/2, MATE1/2-K); AMPK-linked signaling; hepatic gluconeogenesis down-regulation; intestinal GLP-1 modulation.
Pharmacogenomic Effects/ADRs
Variation in SLC22A1 (OCT1) and related transporters may influence response and GI tolerability; transporter inhibitors (e.g., cimetidine) can increase exposure.
3. Interactions
Drug Interactions
- Cimetidine, dolutegravir, ranolazine (OCT/MATE inhibition): ↑ metformin exposure—monitor and consider dose limits.
- Iodinated contrast media (procedural): temporary interruption in at-risk patients; resume after renal function reassessed.
- Carbonic anhydrase inhibitors and conditions predisposing to hypoxia or dehydration: ↑ acidosis risk—use caution.
Food Interactions
Take with meals to reduce GI effects; alcohol increases lactic-acidosis risk.
4. Categories
ATC Codes
A10BA02 (metformin, plain; biguanides)
Drug Categories
Antihyperglycemic; Biguanide; Small molecule
Chemical Taxonomy
Dimethylbiguanide; strong polycation at physiological pH; hydrophilic
Affected organisms
Humans (therapeutic use)
5. Chemical Identifiers
UNII
9100L32L2N (metformin base); 786Z46389E (metformin hydrochloride)
CAS number
657-24-9 (base); 1115-70-4 (hydrochloride)
InChI Key
XZWYZXLIPXDOLR-UHFFFAOYSA-N (base)
InChI
InChI=1S/C4H11N5/c1-9(2)4(7)8-3(5)6/h1-2H3,(H5,5,6,7,8)
IUPAC Name
1-[(diaminomethylidene)amino]-N,N-dimethylmethanimidamide
SMILES
CN(C)C(=N)N=C(N)N
6. References
- DrugBank Online: Metformin (DB00331) — identification, half-life notes, distribution compartment. DrugBank
- PubChem Compound: Metformin (CID 4091) — identifiers, UNII mapping, base properties. PubChem+1
- IUPHAR/BPS Guide to Pharmacology — CAS 657-24-9, SMILES, InChI, InChIKey, cross-refs (ChEMBL, ChEBI, DrugBank). guidetopharmacology.org
- WHO ATC/DDD resources — A10BA02 (metformin), DDD context; EML listing (first added 1997). Essential Medicines List+3atcddd.fhi.no+3World Health Organization+3
- StatPearls (Metformin) — Vd (~654 ± 358 L for 850 mg IR), food effect on absorption, clinical pharmacology. NCBI
- DailyMed / FDA labels (multiple products) — boxed warning: lactic acidosis, interaction/renal guidance, ER/IR notes. DailyMed+3DailyMed+3DailyMed+3
- Mechanism reviews — hepatic Complex I → AMPK activation; gut-mediated actions. Lippincott Journals+3PMC+3MDPI+3
- MALA reviews — distribution, protein binding, role of hemodialysis in severe toxicity. NCBI+1
- DrugBank Salt entry — Metformin hydrochloride identifiers (CAS 1115-70-4; UNII 786Z46389E). DrugBank

I am pursuing MBA in pharmaceutical management from NIPER Hyderabad with a strong academic record and proven success in national-level pharmacy entrance exams. I secured AIR 61 in NIPER 2024 (MS/M.Pharm) and AIR 27 in NIPER MBA, along with AIR 147 in GPAT 2024 and AIR 907 in GPAT 2023. I also achieved AIR 6 in AIIMS CRE-2025 for Drug Store Keeper and was selected as a Pharmacist (AIR 61) for ESIC. Additionally, I was the Runner-Up in Round 2 of the EY Case Study Competition.
At PharmacyFreak.com, I aim to guide future pharmacists through expert content, exam strategies, and insightful resources based on real experience and academic excellence.
Mail- harsh@pharmacyfreak.com
