Table of Contents
Introduction
Azoles are a major class of antifungal drugs widely used for the treatment of systemic and superficial fungal infections. They include imidazoles (e.g., ketoconazole, clotrimazole, miconazole) and triazoles (e.g., fluconazole, itraconazole, voriconazole, posaconazole).
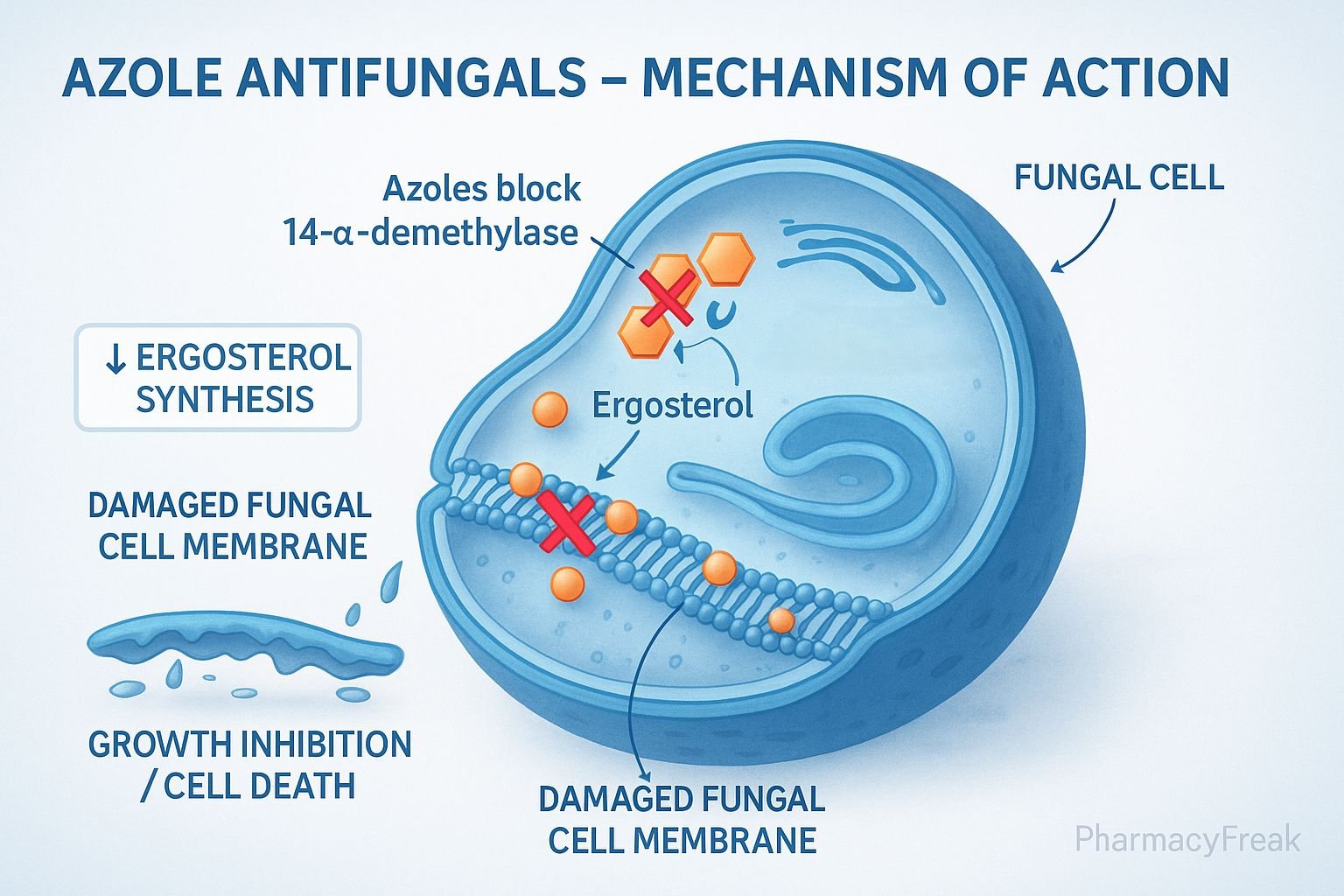
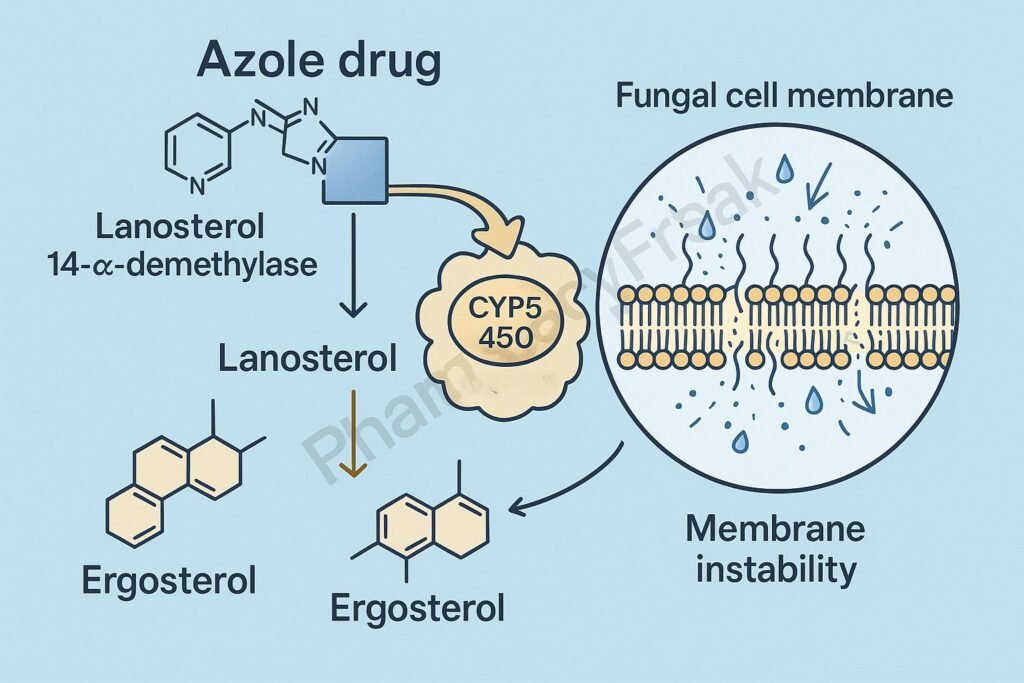
Mechanism of Action of Azole Antifungal Drugs centers on the inhibition of fungal ergosterol synthesis, an essential component of the fungal cell membrane. By targeting the cytochrome P450–dependent enzyme lanosterol 14-α-demethylase, azoles disrupt membrane structure and function, leading to fungal growth inhibition or death.
Mechanism of Action (Step-wise)
1. Target: Fungal Ergosterol Biosynthesis
- Mechanism:
- Fungal cell membranes require ergosterol (analogous to cholesterol in human cells) for integrity, fluidity, and function.
- Azoles inhibit lanosterol 14-α-demethylase, a cytochrome P450 (CYP51) enzyme that converts lanosterol → ergosterol.
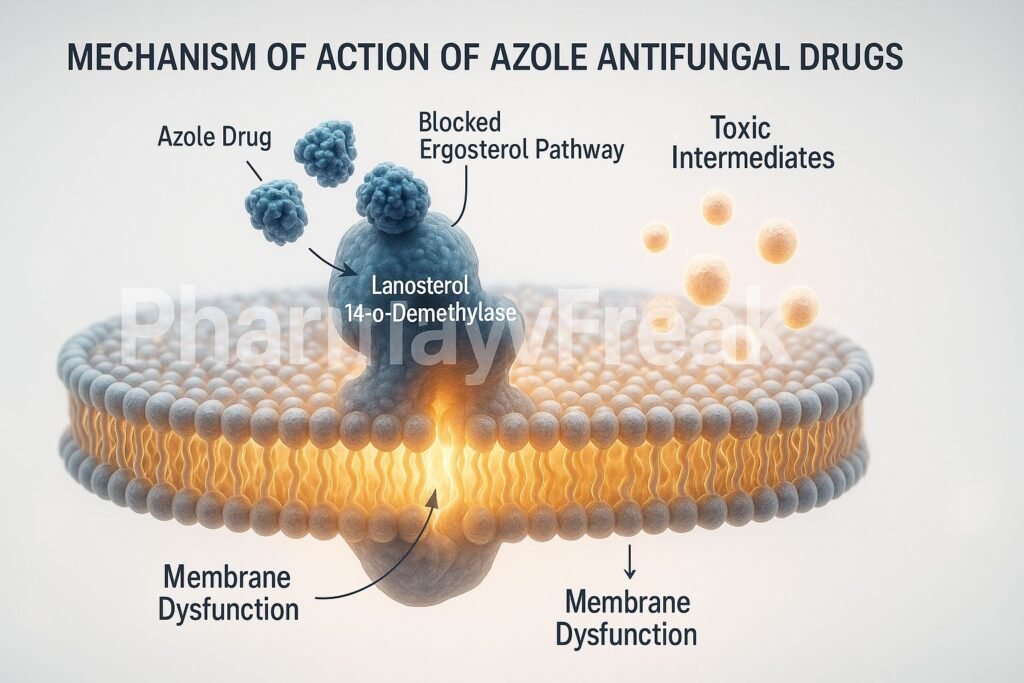
- This enzyme inhibition blocks the demethylation step, leading to accumulation of toxic sterol intermediates and depletion of ergosterol.
- Result:
- Impaired membrane synthesis.
- Increased permeability.
- Inhibition of fungal growth (fungistatic effect).
2. Structural and Functional Disruption of Cell Membrane
- Mechanism:
- Reduced ergosterol weakens the fungal cell membrane, causing leakage of essential intracellular ions and metabolites.
- Altered permeability interferes with nutrient uptake and enzyme localization.
- Effect:
- Fungal cells become fragile and unable to maintain osmotic balance.
- Leads to growth inhibition or death, depending on drug concentration and fungal species.
3. Selective Toxicity
- Mechanism:
- Azoles preferentially inhibit fungal cytochrome P450 (CYP51) rather than human CYP enzymes.
- However, at higher concentrations (especially imidazoles like ketoconazole), cross-inhibition of mammalian P450s can occur → drug interactions and endocrine effects.
- Effect:
- High antifungal specificity at therapeutic doses.
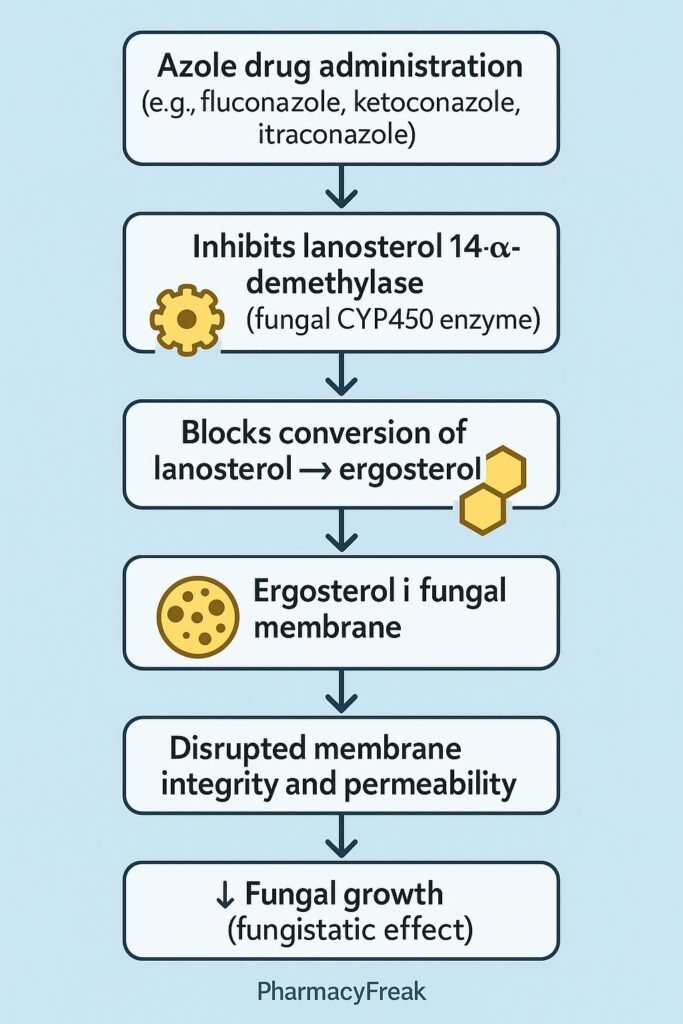
4. Stepwise Summary of Azole Action
| Step | Process Affected | Effect on Fungal Cell |
|---|---|---|
| 1 | Inhibition of lanosterol 14-α-demethylase (CYP51) | ↓ Ergosterol synthesis |
| 2 | Accumulation of 14-α-methyl sterols | Membrane instability |
| 3 | Disruption of membrane structure | Ion leakage and impaired transport |
| 4 | Inhibition of fungal growth | Fungistatic or fungicidal outcome |
Classification of Azoles
| Subclass | Examples | Spectrum / Use | Key Notes |
|---|---|---|---|
| Imidazoles (2 Nitrogen atoms) | Ketoconazole, Clotrimazole, Miconazole | Topical & systemic (limited) | More endocrine toxicity, drug interactions |
| Triazoles (3 Nitrogen atoms) | Fluconazole, Itraconazole, Voriconazole, Posaconazole, Isavuconazole | Broad systemic antifungal use | Greater selectivity, fewer side effects |
Pharmacokinetics
- Absorption:
- Ketoconazole requires an acidic pH (avoid with antacids).
- Fluconazole has excellent oral bioavailability and CNS penetration.
- Itraconazole requires food and low gastric pH.
- Distribution:
- Triazoles distribute widely; fluconazole penetrates CSF.
- Metabolism:
- Hepatic via CYP3A4, except fluconazole (renal excretion).
- Elimination:
- Mainly hepatic (except fluconazole → renal).
Spectrum of Activity
| Drug | Major Fungal Targets |
|---|---|
| Fluconazole | Candida albicans, Cryptococcus neoformans (meningitis) |
| Itraconazole | Histoplasma, Blastomyces, Sporothrix, Dermatophytes |
| Voriconazole | Aspergillus, Fusarium, Candida (resistant species) |
| Posaconazole | Mucorales (Zygomycosis), Aspergillus |
| Ketoconazole | Dermatophytes, Malassezia, limited systemic use |
Adverse Effects
- Common: Nausea, vomiting, abdominal pain, rash.
- Hepatotoxicity: Most azoles can cause elevated liver enzymes or hepatic injury.
- Endocrine effects: Ketoconazole inhibits human steroid synthesis → gynecomastia, decreased libido.
- Drug interactions:
- Potent inhibitors of CYP3A4 → increased plasma levels of warfarin, cyclosporine, statins, etc.
- QT prolongation: Some triazoles (e.g., voriconazole) can prolong QT interval.
Comparative Table: Imidazoles vs Triazoles
| Parameter | Imidazoles | Triazoles |
|---|---|---|
| Nitrogen atoms in ring | 2 | 3 |
| Examples | Ketoconazole, Clotrimazole | Fluconazole, Itraconazole |
| Spectrum | Narrow | Broad |
| Toxicity | More endocrine & hepatic | Lower, more selective |
| Use | Topical/systemic (limited) | Systemic (preferred) |
| CYP inhibition | Strong | Moderate |
MCQs
1. Azoles act by inhibiting:
a) β-glucan synthesis
b) Squalene epoxidase
c) Lanosterol 14-α-demethylase
d) RNA polymerase
Answer: c) Lanosterol 14-α-demethylase
2. The key component of the fungal membrane targeted by azoles is:
a) Cholesterol
b) Ergosterol
c) Phosphatidylcholine
d) Sphingomyelin
Answer: b) Ergosterol
3. Azole antifungals interfere with:
a) DNA replication
b) Protein synthesis
c) Sterol synthesis
d) Cell wall synthesis
Answer: c) Sterol synthesis
4. The enzyme inhibited by azoles is part of which system?
a) Cytochrome P450 system
b) Glycolytic pathway
c) Electron transport chain
d) Nucleotide synthesis pathway
Answer: a) Cytochrome P450 system
5. Ketoconazole inhibits human CYP enzymes, leading to:
a) Adrenal suppression and gynecomastia
b) Hypotension
c) Bronchospasm
d) Diuresis
Answer: a) Adrenal suppression and gynecomastia
6. Which azole is safest for fungal meningitis?
a) Ketoconazole
b) Fluconazole
c) Itraconazole
d) Posaconazole
Answer: b) Fluconazole
7. Azoles are classified as:
a) Fungicidal
b) Fungistatic
c) Bactericidal
d) Antiviral
Answer: b) Fungistatic
8. Azoles can increase plasma concentration of which drug?
a) Warfarin
b) Furosemide
c) Digoxin
d) Amoxicillin
Answer: a) Warfarin
9. Voriconazole is preferred in the treatment of:
a) Candida albicans
b) Aspergillus infection
c) Dermatophytes
d) Mucorales infection
Answer: b) Aspergillus infection
10. Posaconazole is uniquely effective against:
a) Candida
b) Mucormycosis
c) Cryptococcus
d) Blastomyces
Answer: b) Mucormycosis
FAQs
Q1. What is the main target of azole antifungal drugs?
The enzyme lanosterol 14-α-demethylase, essential for converting lanosterol to ergosterol in fungal cell membranes.
Q2. Why are triazoles preferred over imidazoles?
They are more selective for fungal CYP enzymes, have broader spectra, and cause fewer hormonal side effects.
Q3. Can azoles be used in immunocompromised patients?
Yes, triazoles like fluconazole, itraconazole, and voriconazole are often used prophylactically in such patients.
Q4. Why should azoles be used cautiously with other drugs?
They inhibit cytochrome P450 enzymes, increasing plasma levels of many co-administered drugs.
Q5. What is the difference between fluconazole and itraconazole?
Fluconazole penetrates CSF and treats cryptococcal meningitis, while itraconazole covers dimorphic fungi like Histoplasma and Blastomyces.
References

I am pursuing MBA in pharmaceutical management from NIPER Hyderabad with a strong academic record and proven success in national-level pharmacy entrance exams. I secured AIR 61 in NIPER 2024 (MS/M.Pharm) and AIR 27 in NIPER MBA, along with AIR 147 in GPAT 2024 and AIR 907 in GPAT 2023. I also achieved AIR 6 in AIIMS CRE-2025 for Drug Store Keeper and was selected as a Pharmacist (AIR 61) for ESIC. Additionally, I was the Runner-Up in Round 2 of the EY Case Study Competition.
At PharmacyFreak.com, I aim to guide future pharmacists through expert content, exam strategies, and insightful resources based on real experience and academic excellence.
Mail- harsh@pharmacyfreak.com