Table of Contents
Introduction
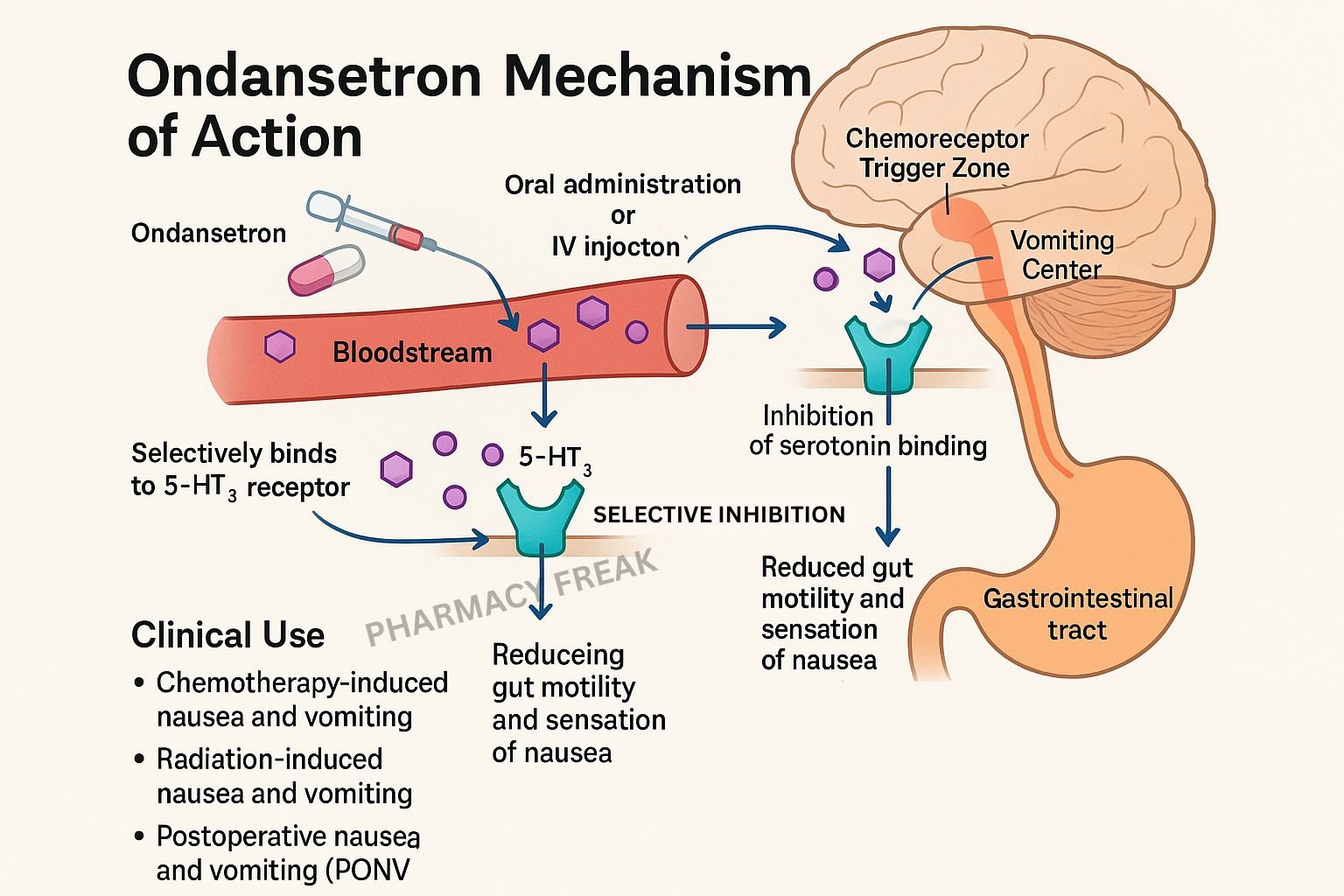
Ondansetron is a highly selective 5‑hydroxytryptamine 3 (5‑HT₃) receptor antagonist used primarily as an antiemetic. It prevents nausea and vomiting triggered by chemotherapy, radiotherapy, surgery, and gastrointestinal irritants. It acts both peripherally in the gastrointestinal tract and centrally in the brainstem’s vomiting centers.
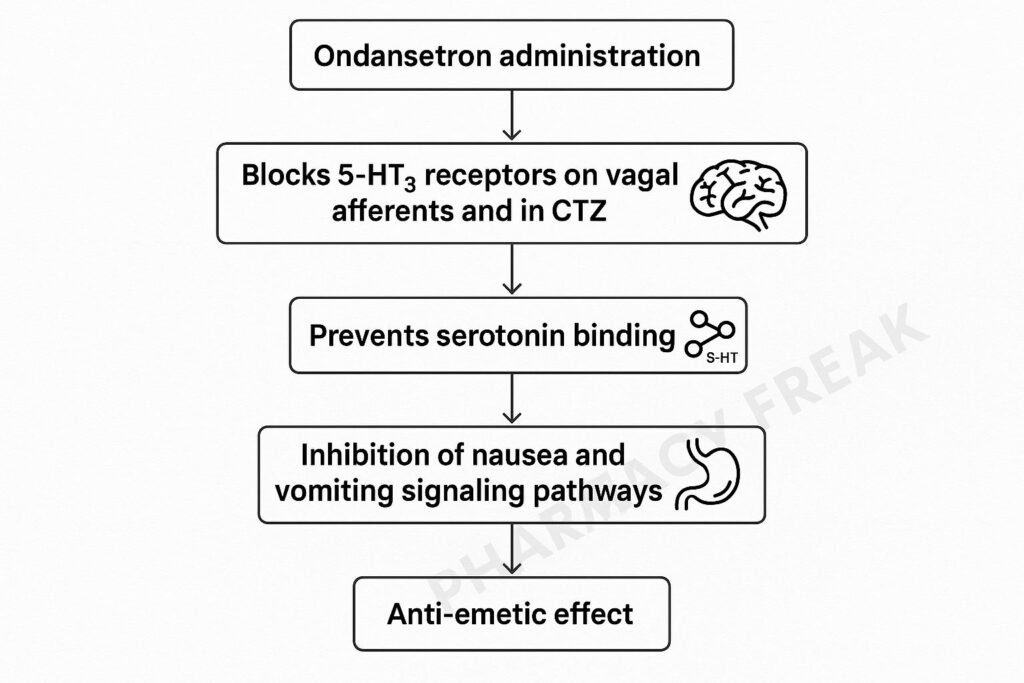
Step‑by‑Step Mechanism of Action
- Selective 5‑HT₃ receptor blockade
Ondansetron binds competitively to 5‑HT₃ receptors, preventing serotonin from activating these ionotropic receptors. - Peripheral action in the gut
Chemotherapy and other emetogenic stimuli cause enterochromaffin cells in the gastrointestinal mucosa to release serotonin, which stimulates vagal afferent nerves via 5‑HT₃ receptors. Ondansetron blocks this pathway, preventing initiation of the vomiting reflex. - Central action in vomiting centers
It also blocks 5‑HT₃ receptors in the area postrema (chemoreceptor trigger zone) and nucleus tractus solitarius, preventing serotonin-mediated activation of the vomiting centers in the brainstem. - Ion channel inhibition
As a ligand-gated non-selective cation channel, 5‑HT₃ receptor blockade prevents sodium and potassium influx, halting neuronal depolarization that would otherwise propagate emetic signaling.
Pharmacokinetic Parameters
| Parameter | Ondansetron Values |
|---|---|
| Bioavailability | ~60% (oral) |
| Protein binding | 70–76% |
| Metabolism | Liver (CYP3A4, CYP1A2, CYP2D6) |
| Half-life | ~5.7 hours |
| Excretion | Renal |
| Clearance | Decreased in severe renal impairment, but no dose adjustment usually needed |
Clinical Uses
- Prevention and treatment of chemotherapy-induced nausea and vomiting (CINV)
- Prevention of postoperative nausea and vomiting (PONV)
- Management of nausea in radiotherapy, gastroenteritis, and migraine
- Off-label use in cyclic vomiting syndrome and carcinoid diarrhea
Adverse Effects
- Common: headache, constipation, diarrhea, dizziness
- Serious: QT-interval prolongation, especially with high IV doses
- Minimal extrapyramidal effects due to lack of dopaminergic blockade
Comparative Analysis
Ondansetron is part of the “setron” class of antiemetics, sharing its mechanism with drugs like granisetron and palonosetron. Compared to palonosetron, which has a half-life of ~40 hours, ondansetron has a shorter duration (~6 hours), making it suitable for short-term use and often requiring multiple doses for prolonged coverage.
MCQs
1. Ondansetron primarily antagonizes which receptor subtype?
a) 5‑HT₁A b) 5‑HT₂A c) 5‑HT₃ d) D₂
Answer: c) 5‑HT₃
2. Which action best describes ondansetron’s peripheral effect?
a) Enhances serotonin release
b) Blocks vagal nerve 5‑HT₃ receptors
c) Inhibits dopamine D₂ receptors
d) Activates NK‑1 receptors
Answer: b) Blocks vagal nerve 5‑HT₃ receptors
3. Ondansetron blocks serotonin in which central structure?
a) Hippocampus b) Area postrema c) Basal ganglia d) Amygdala
Answer: b) Area postrema
4. What ion flux is inhibited by 5‑HT₃ blockade?
a) Calcium b) Chloride c) Sodium/potassium d) Magnesium
Answer: c) Sodium/potassium
5. What is the oral bioavailability of ondansetron?
a) 20% b) 40% c) 60% d) 80%
Answer: c) 60%
6. Ondansetron is primarily metabolized by:
a) CYP2C9 b) CYP3A4, CYP1A2, CYP2D6 c) CYP2E1 d) CYP2C19
Answer: b) CYP3A4, CYP1A2, CYP2D6
7. Typical half-life of ondansetron is ~:
a) 2 hrs b) 6 hrs c) 12 hrs d) 24 hrs
Answer: b) 6 hrs
8. Most serious cardiac side effect is:
a) Bradycardia b) QT prolongation c) Heart block d) SVT
Answer: b) QT prolongation
9. Ondansetron lacks which receptor binding?
a) Histamine b) Dopamine c) Muscarinic d) All
Answer: d) All
10. Recommended use in pregnancy emesis:
a) Category X b) Off-label for morning sickness c) Teratogenic d) Contraindicated
Answer: b) Off-label for morning sickness
11. Ondansetron’s protein binding is approx:
a) 10% b) 50% c) 75% d) 95%
Answer: c) 75%
12. In renal failure, clearance:
a) Doubles b) Halved c) Unchanged d) Triples
Answer: b) Halved
13. It is the first-line therapy for:
a) Motion sickness b) Chemotherapy-induced emesis c) Migraine d) Vertigo
Answer: b) Chemotherapy-induced emesis
14. Ondansetron is contraindicated in:
a) Asthma b) Late pregnancy c) Congenital long QT d) Hypertension
Answer: c) Congenital long QT
15. Compared to palonosetron, ondansetron has:
a) Longer half-life b) Greater receptor affinity c) Shorter half-life d) Irreversible binding
Answer: c) Shorter half-life
FAQs
1. Can ondansetron be used during pregnancy?
Yes, often prescribed off-label for morning sickness after first-line options fail.
2. Does ondansetron require ECG monitoring?
Recommended in patients with known QT prolongation or using other QT‑extending drugs.
3. Can ondansetron be taken orally or IV?
Both routes are effective, with rapid onset of action.
4. Why doesn’t ondansetron cause sedation?
Because it does not act on histaminic, muscarinic, or dopaminergic receptors.
5. Can ondansetron interact with chemotherapy drugs?
Yes—it’s sometimes used synergistically with NK‑1 antagonists for enhanced antiemetic effect.
References
- Goodman & Gilman’s The Pharmacological Basis of Therapeutics, 13th Edition.
- KD Tripathi. Essentials of Medical Pharmacology, 8th Edition.
- StatPearls: Antiemetics, Selective 5‑HT₃ Antagonists
- StatPearls: Ondansetron overview
- PubMed: Comparative efficacy of serotonin (5‑HT₃) receptor antagonists
- Frontiers in Pharmacology: Ondansetron and chemotherapy-induced antiemesis
- Clinical Pharmacokinetics of Ondansetron

I am pursuing MBA in pharmaceutical management from NIPER Hyderabad with a strong academic record and proven success in national-level pharmacy entrance exams. I secured AIR 61 in NIPER 2024 (MS/M.Pharm) and AIR 27 in NIPER MBA, along with AIR 147 in GPAT 2024 and AIR 907 in GPAT 2023. I also achieved AIR 6 in AIIMS CRE-2025 for Drug Store Keeper and was selected as a Pharmacist (AIR 61) for ESIC. Additionally, I was the Runner-Up in Round 2 of the EY Case Study Competition.
At PharmacyFreak.com, I aim to guide future pharmacists through expert content, exam strategies, and insightful resources based on real experience and academic excellence.
Mail- harsh@pharmacyfreak.com