Table of Contents
1. Identification
Summary
Nonsteroidal anti-inflammatory drugs (NSAIDs) inhibit cyclo-oxygenase (COX-1/COX-2) to reduce prostaglandin and thromboxane synthesis, producing analgesic, antipyretic, and anti-inflammatory effects; aspirin uniquely irreversibly acetylates COX.
Brand Names
Not applicable at class level (examples span multiple brands and generics).
Name
NSAIDs (Nonsteroidal Anti-inflammatory Drugs)
Background
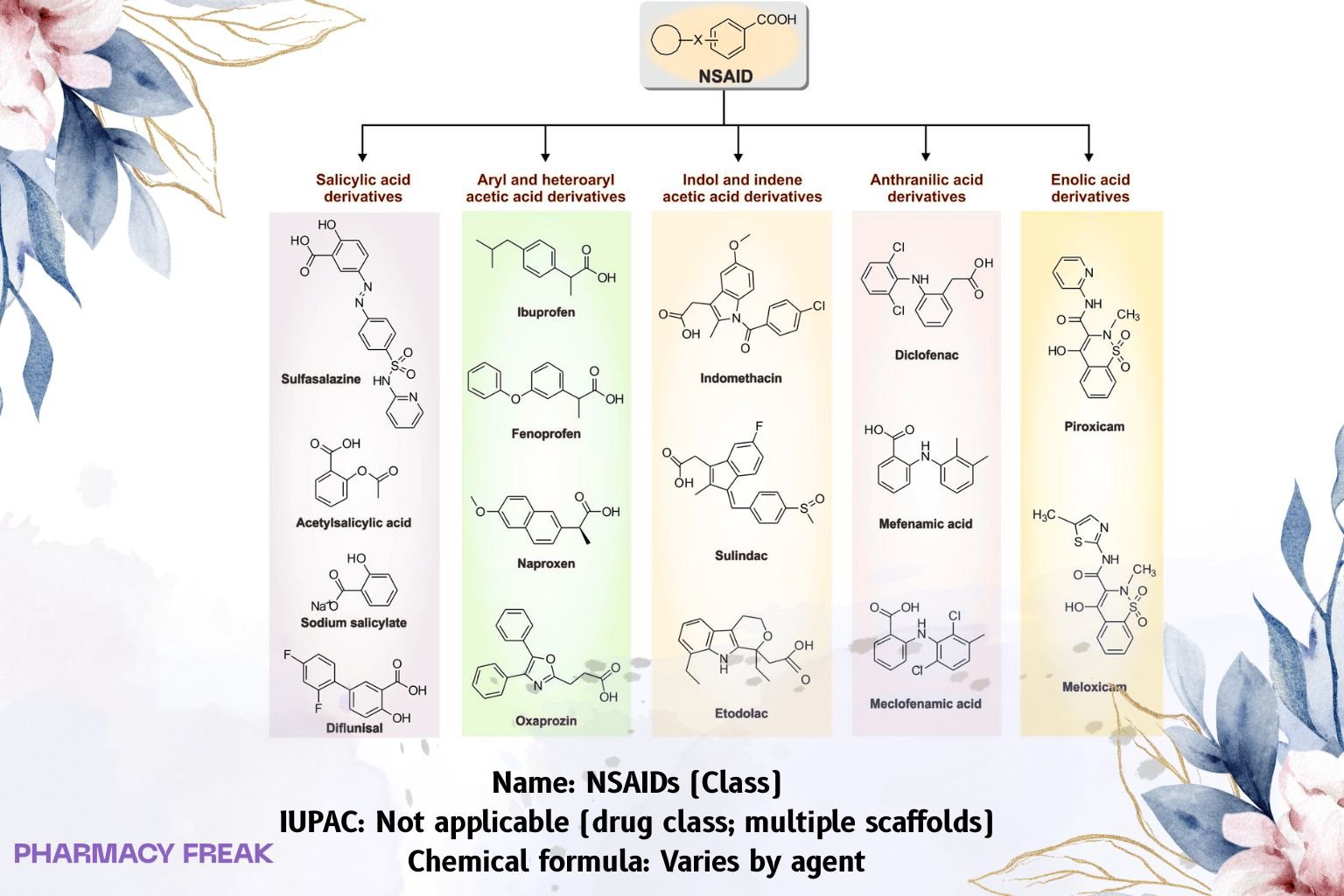
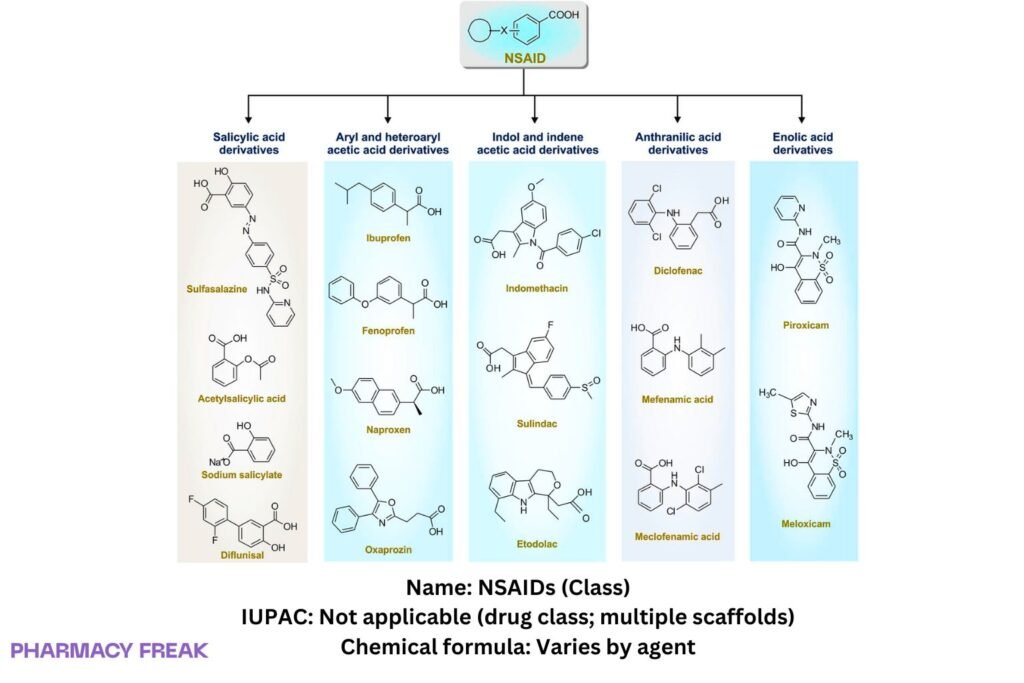
Subclasses:
• Salicylates (e.g., aspirin) — irreversible acetylation
• Arylpropionic acids (e.g., ibuprofen, naproxen) — reversible, COX-1/2
• Arylacetic acids (e.g., diclofenac, indomethacin, ketorolac)
• Enolic acids/oxicams (e.g., piroxicam, meloxicam)
• Fenamates (e.g., mefenamic acid)
• Selective COX-2 inhibitors (coxibs) (e.g., celecoxib, etoricoxib)
Modality
Small molecules
Groups
Approved; prescription/OTC (jurisdiction and product dependent)
Structure
Weight
Varies by agent
Chemical Formula
Varies by agent
Synonyms
Nonsteroidal anti-inflammatory agents; COX inhibitors
External IDs
ATC family: M01A (subgroups per subclass)
2. Pharmacology
Indication
Symptomatic treatment of pain, fever, and inflammation (musculoskeletal disorders, dysmenorrhea, dental pain); migraine (some agents); gout flares; PDA closure in neonates (indomethacin/ibuprofen IV); cardiovascular prevention at low-dose aspirin (antiplatelet).
Associated Conditions
Osteoarthritis, rheumatoid arthritis, spondyloarthritides, peri-operative pain (short-course ketorolac), headache disorders, soft-tissue injury.
Associated Therapies
With gastroprotection (PPI) in GI-risk patients; with acetaminophen in multimodal analgesia; low-dose aspirin often continued for cardioprotection (manage timing with other NSAIDs).
Contraindications & Blackbox Warnings
Class boxed warnings (non-aspirin NSAIDs and COX-2 selective): ↑ risk of serious cardiovascular thrombotic events (MI, stroke); ↑ risk of serious GI bleeding, ulceration, perforation.
Contraindications: peri-operative pain in CABG, active GI bleeding/ulcer, prior NSAID-induced asthma/urticaria, severe renal failure without monitoring. Pregnancy: avoid from 20 weeks (fetal renal dysfunction/oligohydramnios) and especially after 30 weeks (ductus arteriosus closure).
Pharmacodynamics
COX inhibition → ↓ PGE₂/PGI₂ (analgesia, antipyresis, ↓ inflammation); COX-1 gastric/platelet inhibition explains GI bleeding and antiplatelet effects. Aspirin irreversibly blocks platelet COX-1 for lifespan; others are reversible.
Mechanism of action
Competitive (reversible) blockade of COX-1/COX-2 active sites (all non-aspirin NSAIDs); acetylation of a serine residue by aspirin (irreversible) → prevents arachidonic-acid conversion to prostanoids.
Absorption
Rapid oral absorption for most; peak 0.5–3 h; food may delay tₘₐₓ without major AUC change.
Volume of distribution
Moderate; highly albumin-bound (often >95%) with acidic scaffolds.
Protein binding
High for most arylacetic/arylpropionic members; celecoxib moderately high.
Metabolism
Predominantly hepatic (CYP2C9 for many; glucuronidation common). Aspirin → salicylate by esterases.
Route of elimination
Renal (metabolites ± unchanged) ± biliary; half-life ranges: short (ibuprofen 2–4 h) to long (piroxicam ~45–50 h).
Half-life
Agent-specific; sustained-release and long-t½ oxicams permit once-daily dosing.
Clearance
Hepatic metabolism; renal impairment chiefly affects metabolites/salicylate; dose adjust where labeled.
Adverse Effects
GI: dyspepsia, ulcer, bleeding; renal: AKI, sodium/water retention, ↑ BP; CV: MI/stroke risk (non-aspirin); hematologic: platelet inhibition (aspirin); hypersensitivity/bronchospasm (AERD); hepatic: transaminitis (diclofenac signal); CNS: headache, dizziness.
Toxicity
Overdose: tinnitus (salicylates), metabolic derangements, CNS symptoms; treat supportively (alkalinize urine for salicylate). Non-aspirin NSAIDs: mainly GI/renal/CNS toxicity.
Pathways
Arachidonic-acid cascade blockade at COX; downstream ↓ prostaglandins/thromboxanes.
Pharmacogenomic Effects/ADRs
CYP2C9 poor metabolizers: ↑ exposure to several NSAIDs (e.g., celecoxib); clinical impact drug-specific.
3. Interactions
Drug Interactions
- Anticoagulants/antiplatelets/SSRIs/SNRIs → ↑ bleeding risk
- ACEI/ARB + diuretic (“triple whammy”) → ↑ AKI risk
- Lithium → ↓ renal clearance (↑ levels)
- Methotrexate → ↓ renal clearance (↑ toxicity), especially high doses
- CYP2C9 inhibitors/inducers → exposure changes (celecoxib, diclofenac, ibuprofen, naproxen)
- Low-dose aspirin: ibuprofen taken first may block aspirin’s irreversible platelet access—separate dosing (aspirin first; delay ibuprofen)
Food Interactions
Food may delay absorption; some labels prefer dosing with food to reduce GI upset.
4. Categories
ATC Codes
M01A (anti-inflammatory and antirheumatic products, non-steroids); specific subgroups per subclass (e.g., M01AE propionic acids, M01AB acetic acids, M01AC oxicams, M01AH COX-2 inhibitors).
Drug Categories
Analgesic; Antipyretic; Anti-inflammatory; Antiplatelet (aspirin, low dose)
Chemical Taxonomy
Predominant arylpropionic and arylacetic carboxylic acids, enolic acids (oxicams), and diarylheterocycles (coxibs); weak acids with high albumin binding.
Affected organisms
Humans (therapeutic use)
5. Chemical Identifiers
UNII / CAS / InChI / SMILES
Not applicable at class level (agent-specific).
Representative scaffolds: arylpropionic acid (Ar–CH(CH₃)–CO₂H); diaryl-pyrazole/pyrazoline (coxibs).
6. References
Goodman & Gilman’s The Pharmacological Basis of Therapeutics — NSAIDs and coxibs chapter.
Katzung & Trevor’s Basic & Clinical Pharmacology — Nonopioid analgesics, antipyretics, NSAIDs.
Hochberg et al., rheumatology and osteoarthritis guidelines (GI/CV risk stratification).
FDA class labeling for non-aspirin NSAIDs and aspirin (boxed warnings; pregnancy precautions).
Up-to-date pharmaceutics reviews on COX biology, platelet effects, and drug–drug interactions.

I am pursuing MBA in pharmaceutical management from NIPER Hyderabad with a strong academic record and proven success in national-level pharmacy entrance exams. I secured AIR 61 in NIPER 2024 (MS/M.Pharm) and AIR 27 in NIPER MBA, along with AIR 147 in GPAT 2024 and AIR 907 in GPAT 2023. I also achieved AIR 6 in AIIMS CRE-2025 for Drug Store Keeper and was selected as a Pharmacist (AIR 61) for ESIC. Additionally, I was the Runner-Up in Round 2 of the EY Case Study Competition.
At PharmacyFreak.com, I aim to guide future pharmacists through expert content, exam strategies, and insightful resources based on real experience and academic excellence.
Mail- harsh@pharmacyfreak.com