Table of Contents
Introduction
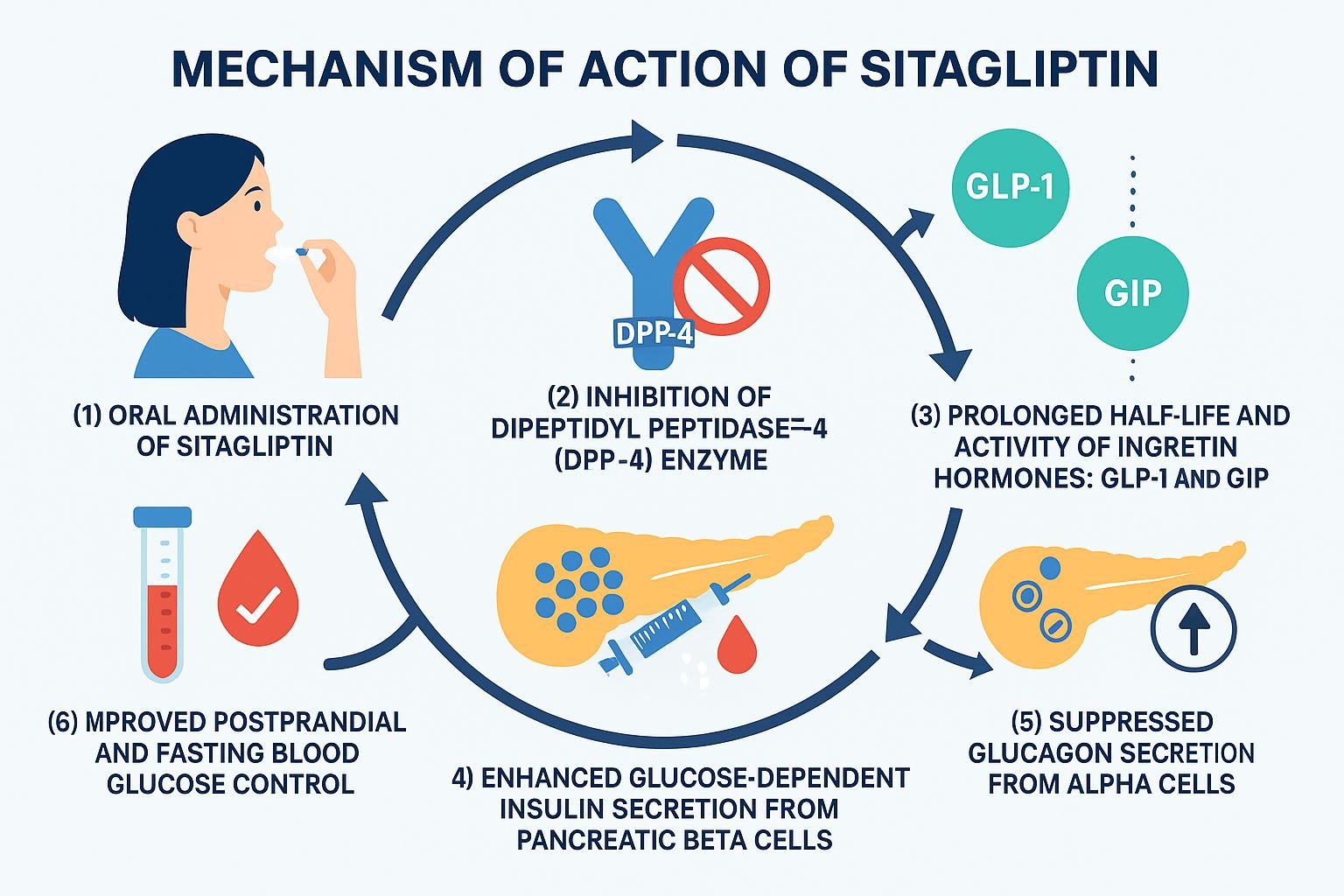
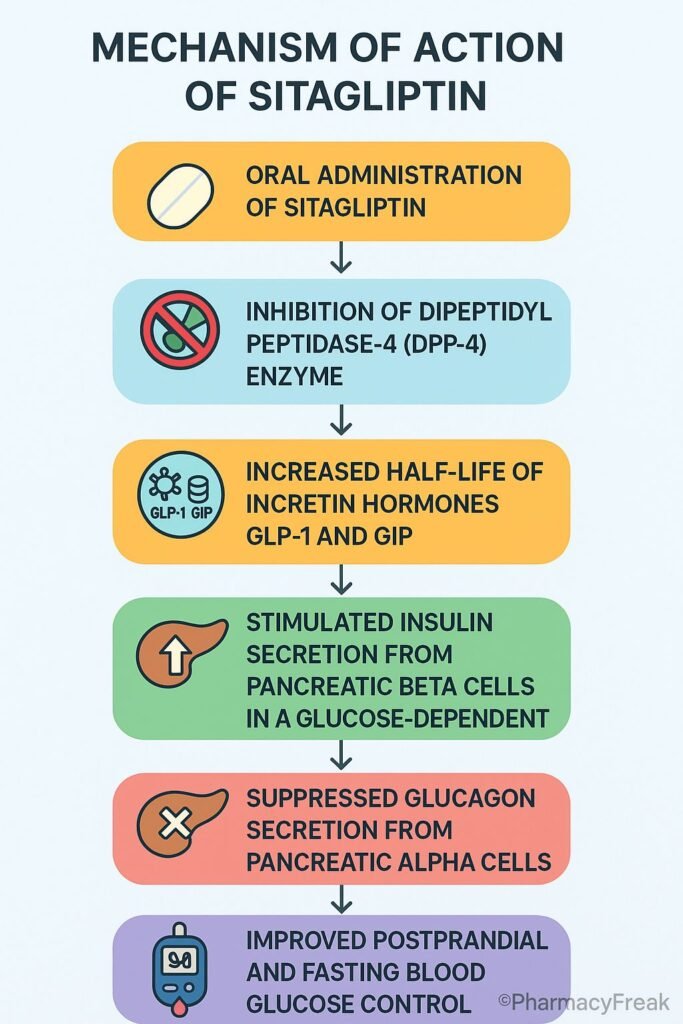
Sitagliptin is an oral dipeptidyl peptidase‑4 (DPP‑4) inhibitor used to manage type 2 diabetes mellitus (T2DM). It improves glycemic control by enhancing incretin hormone activity, leading to increased insulin secretion and decreased glucagon release in a glucose-dependent manner.
Step-by-Step Mechanism of Action
- Inhibition of DPP‑4 enzyme
Sitagliptin selectively inhibits the DPP‑4 enzyme, which normally degrades incretin hormones. - Increased incretin levels
The inhibition of DPP‑4 prolongs the activity of glucagon-like peptide‑1 (GLP‑1) and glucose-dependent insulinotropic polypeptide (GIP). - Enhanced insulin secretion
Elevated incretin levels stimulate insulin release from pancreatic β-cells in a glucose-dependent manner. - Suppression of glucagon
GLP‑1 also suppresses glucagon secretion from pancreatic α-cells, reducing hepatic glucose output. - Improved glycemic control
These actions together lead to reduced fasting and postprandial blood glucose without causing hypoglycemia.
Pharmacokinetic Parameters
| Parameter | Value |
|---|---|
| Route | Oral |
| Bioavailability | ~87% |
| Protein Binding | ~38% |
| Metabolism | Minimal; primarily renal excretion |
| Half-life | ~12 hours |
| Excretion | Primarily renal (unchanged drug) |
Clinical Uses
- Monotherapy or add-on therapy in type 2 diabetes mellitus
- Often used in combination with metformin, sulfonylureas, or insulin
Adverse Effects
- Nasopharyngitis
- Headache
- Upper respiratory tract infections
- Rare: pancreatitis, hypersensitivity reactions, joint pain
Comparative Analysis
| Agent | Mechanism | Dosing | Hypoglycemia Risk |
|---|---|---|---|
| Sitagliptin | DPP‑4 inhibitor | Once daily | Low |
| Saxagliptin | DPP‑4 inhibitor | Once daily | Low |
| Liraglutide | GLP‑1 receptor agonist | Once daily | Moderate |
| Metformin | Reduces hepatic glucose production | Twice daily | Minimal |
MCQs
- Sitagliptin inhibits which enzyme?
a) SGLT2 b) DPP‑4 c) α-glucosidase d) CYP3A4
Answer: b) DPP‑4 - Sitagliptin’s primary mechanism involves:
a) Enhancing insulin sensitivity b) Increasing insulin secretion via incretins c) Inhibiting glucose absorption d) Stimulating glucagon
Answer: b) Increasing insulin secretion via incretins - GLP‑1 and GIP are degraded by:
a) CYP enzymes b) Amylase c) DPP‑4 d) Pepsin
Answer: c) DPP‑4 - Sitagliptin lowers blood glucose by:
a) Inhibiting SGLT2 b) Blocking insulin degradation c) Increasing hepatic glucose output d) Enhancing insulin and reducing glucagon
Answer: d) Enhancing insulin and reducing glucagon - Risk of hypoglycemia with sitagliptin monotherapy is:
a) High b) Moderate c) Low d) Guaranteed
Answer: c) Low - Excretion route for sitagliptin is primarily:
a) Biliary b) Renal c) Pulmonary d) Salivary
Answer: b) Renal - Dosing adjustment is required in:
a) Hepatic impairment b) Mild asthma c) Renal impairment d) Gout
Answer: c) Renal impairment - Sitagliptin’s main benefit over sulfonylureas is:
a) Once-weekly dosing b) Weight gain c) No hypoglycemia d) Stronger glucose control
Answer: c) No hypoglycemia - It increases levels of:
a) Cortisol b) Insulin-independent glucose uptake c) GLP‑1 and GIP d) Adrenaline
Answer: c) GLP‑1 and GIP - Common side effect of sitagliptin is:
a) Pancreatitis b) Nasopharyngitis c) Weight loss d) Diarrhea
Answer: b) Nasopharyngitis
FAQs
1. Does sitagliptin cause hypoglycemia?
Not when used alone; risk increases with insulin or sulfonylureas.
2. Can it be used in renal impairment?
Yes, but dose adjustment is required based on creatinine clearance.
3. Does it aid in weight loss?
It is generally weight neutral.
4. Is it safe in elderly patients?
Yes, but renal function should be monitored.
5. How long does it take to show effect?
Glycemic improvement is usually observed within a few weeks of initiation.
References

I am pursuing MBA in pharmaceutical management from NIPER Hyderabad with a strong academic record and proven success in national-level pharmacy entrance exams. I secured AIR 61 in NIPER 2024 (MS/M.Pharm) and AIR 27 in NIPER MBA, along with AIR 147 in GPAT 2024 and AIR 907 in GPAT 2023. I also achieved AIR 6 in AIIMS CRE-2025 for Drug Store Keeper and was selected as a Pharmacist (AIR 61) for ESIC. Additionally, I was the Runner-Up in Round 2 of the EY Case Study Competition.
At PharmacyFreak.com, I aim to guide future pharmacists through expert content, exam strategies, and insightful resources based on real experience and academic excellence.
Mail- harsh@pharmacyfreak.com