Table of Contents
1. Identification
Summary
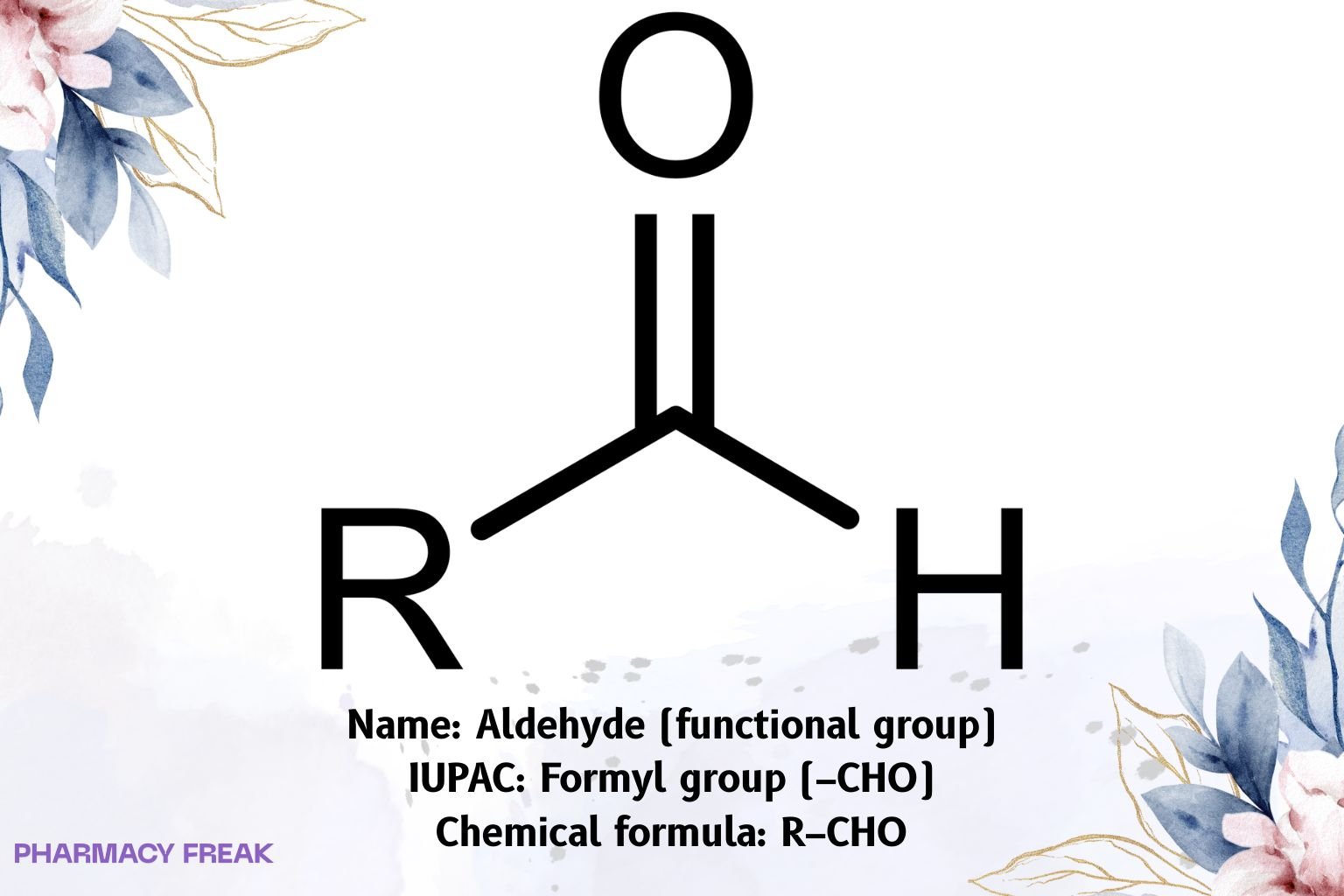
Aldehyde is a carbonyl functional group in which the carbonyl carbon is bonded to one hydrogen and one carbon (R–CHO). It is a terminal, sp², trigonal-planar carbonyl that is strongly electrophilic; hallmark spectroscopic features include a C=O IR band ~1720–1740 cm⁻¹ (lower with conjugation) and a diagnostic aldehydic C–H doublet near ~2720/2820 cm⁻¹. The ¹H NMR aldehydic proton appears at ~9–10 ppm; ¹³C NMR carbonyl at ~190–205 ppm.
Brand Names
Not applicable.
Name
Aldehyde
Background
Key reactivity: nucleophilic addition to the carbonyl (e.g., hydride/organometallics), acetal formation (with diols under acid), oxidation → carboxylic acids, reduction → primary alcohols, aldol reactions via enol/enolate of the partner carbonyl, Cannizzaro (for non-enolizable aldehydes), Wittig/ylide olefination, cyanohydrin and bisulfite adduct formation.
Modality
Functional group (class of small molecules)
Groups
Endogenous/exogenous chemicals; widespread in natural products and pharmaceuticals
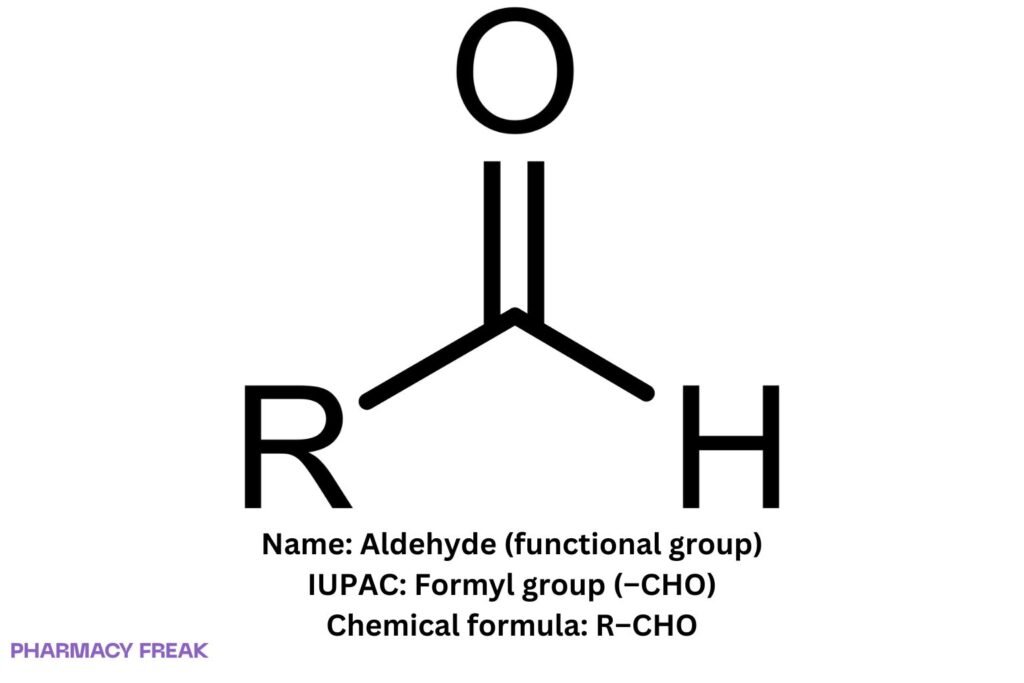
Structure
Weight
Not applicable (class)
Chemical Formula
R–CHO
Synonyms
Formyl group; Alkanal (nomenclature family)
External IDs
Not applicable (class). Examples for context: formaldehyde (CAS 50-00-0), acetaldehyde (CAS 75-07-0), benzaldehyde (CAS 100-52-7)
2. Pharmacology
Indication
Not a therapeutic agent (group descriptor).
Associated Conditions
Occurs in biomolecules and intermediates; relevant in flavor/fragrance chemistry and API synthesis.
Associated Therapies
None (functional group context).
Contraindications & Blackbox Warnings
Not applicable at the group level.
Pharmacodynamics
Chemical behavior: electrophilic C=O accepts nucleophiles; α-hydrogens are acidic (pKₐ ~17) enabling enol/enolate chemistry (aldol/Michael pathways).
Mechanism of action
Polarization of π(C=O) activates carbonyl carbon toward addition; hemiacetal/acetal equilibria under acid catalysis; oxidation/reduction interconvert aldehydes with acids/alcohols.
Absorption
Not applicable (class).
Volume of distribution
Not applicable.
Protein binding
Not applicable.
Metabolism
Chemical interconversions in synthesis/biochemistry as above.
Route of elimination
Not applicable.
Half-life
Not applicable.
Clearance
Not applicable.
Adverse Effects
Not applicable to the functional group per se; safety depends on the specific aldehyde (e.g., formaldehyde is hazardous).
Toxicity
Scaffold-dependent; consult substance-specific safety data.
Pathways
High-yield transformations: ROH oxidation → R–CHO (PCC/Swern/DMP), ozonolysis of alkenes, partial reduction of acid derivatives, Wittig/HWE to alkenes from aldehydes, Cannizzaro (non-enolizable), aldol condensations.
Pharmacogenomic Effects/ADRs
Not applicable.
3. Interactions
Drug Interactions
Chemical: acetalization with diols; Schiff base formation after oxidation/reduction sequences via imine chemistry (with amines) when used as intermediates; bisulfite adducts useful for purification/protection.
Food Interactions
Not applicable (class).
4. Categories
ATC Codes
None (functional group)
Drug Categories
Functional group; Carbonyl compound
Chemical Taxonomy
Terminal carbonyl (R–CHO); sp² planar; IR C=O ~1720–1740 cm⁻¹; aldehydic C–H ~2720/2820 cm⁻¹; keto–enol tautomerism possible via α-deprotonation.
Affected organisms
Not applicable
5. Chemical Identifiers
UNII
Not applicable.
CAS number
Not applicable (class). Examples: formaldehyde 50-00-0, acetaldehyde 75-07-0, benzaldehyde 100-52-7.
InChI Key / InChI
Not applicable (class).
IUPAC Name
Aldehyde functional group (formyl, –CHO)
SMILES
Generic: R–C(=O)H
6. References
IUPAC Gold Book: definitions of aldehyde/formyl group; spectroscopic conventions.
Clayden, Greeves, Warren. Organic Chemistry: aldehyde reactivity (nucleophilic additions, acetals), spectroscopy, and synthesis.
March’s Advanced Organic Chemistry: oxidation/reduction of carbonyls, aldol/Cannizzaro, Wittig/HWE olefination.
Silverstein et al., Spectrometric Identification of Organic Compounds: IR/NMR signatures of aldehydes (C=O, aldehydic C–H, chemical shifts).

I am pursuing MBA in pharmaceutical management from NIPER Hyderabad with a strong academic record and proven success in national-level pharmacy entrance exams. I secured AIR 61 in NIPER 2024 (MS/M.Pharm) and AIR 27 in NIPER MBA, along with AIR 147 in GPAT 2024 and AIR 907 in GPAT 2023. I also achieved AIR 6 in AIIMS CRE-2025 for Drug Store Keeper and was selected as a Pharmacist (AIR 61) for ESIC. Additionally, I was the Runner-Up in Round 2 of the EY Case Study Competition.
At PharmacyFreak.com, I aim to guide future pharmacists through expert content, exam strategies, and insightful resources based on real experience and academic excellence.
Mail- harsh@pharmacyfreak.com