Table of Contents
1. Identification
Summary
Metronidazole is a nitroimidazole antibacterial/antiprotozoal used systemically and topically for susceptible anaerobic infections and select protozoal diseases.
Brand Names
Flagyl, Metrogyl, Metrogel/MetroCream (topical), Vandazole/Nuvessa (vaginal); generics common
Name
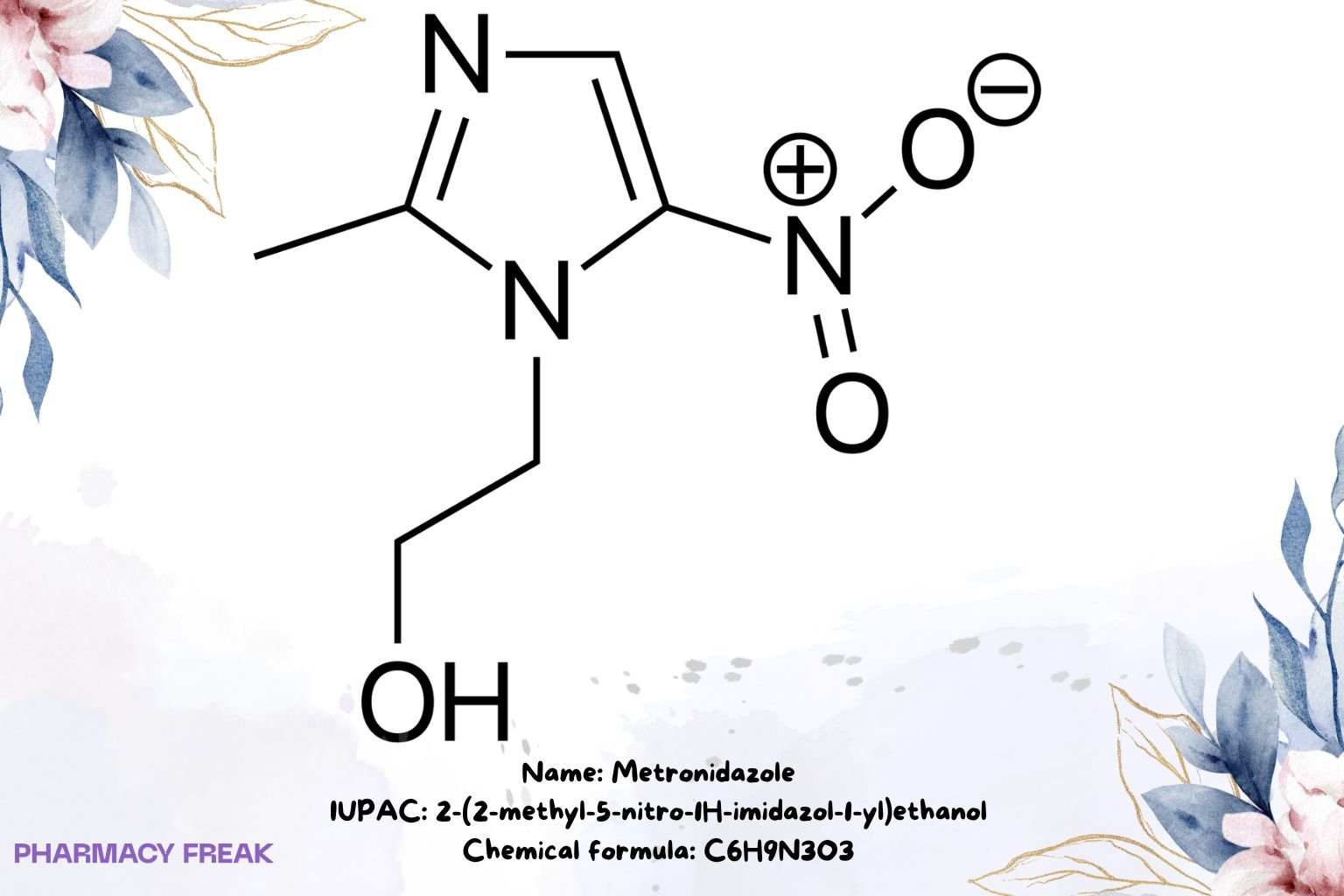
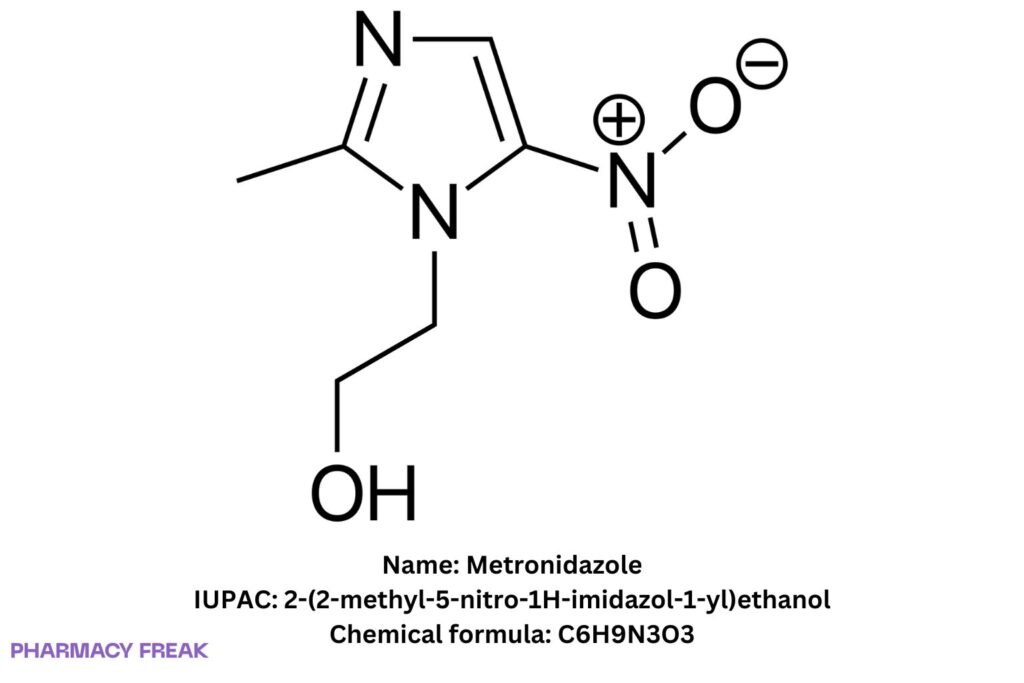
Metronidazole
Background
Synthetic 5-nitroimidazole introduced mid-20th century; oral, IV, topical (dermal), and intravaginal formulations are standard.
Modality
Small molecule
Groups
Approved; prescription
Structure
Weight
~171.15 g/mol (base)
Chemical Formula
C₆H₉N₃O₃
Synonyms
2-(2-methyl-5-nitro-1H-imidazol-1-yl)ethanol; 1-(2-hydroxyethyl)-2-methyl-5-nitroimidazole
External IDs
CAS: 443-48-1; PubChem CID: 4173; UNII: 140QMO216E; ATC (key codes): J01XD01, A01AB17, D06BX01, G01AF01, P01AB01
2. Pharmacology
Indication
Anaerobic bacterial infections (systemic/IV); bacterial vaginosis (oral/vaginal); trichomoniasis; intestinal/hepatic amebiasis; giardiasis; rosacea (topical).
Associated Conditions
Intra-abdominal, gynecologic, skin/soft-tissue, CNS, bone/joint infections due to susceptible anaerobes; dental/oral infections (local use).
Associated Therapies
Used with surgical source control for anaerobic sepsis; combined regimens for mixed aerobic–anaerobic infections per local guidance.
Contraindications & Blackbox Warnings
No boxed warning. Contraindicated with disulfiram within 2 weeks (neuropsychiatric reactions). Avoid alcohol during therapy and for ≥3 days after (reaction risk noted in labeling).
Pharmacodynamics
Prodrug; in anaerobic conditions the nitro group is reduced to reactive intermediates that damage DNA, yielding bactericidal/protozoacidal effects against susceptible organisms.
Mechanism of action
Intracellular electron transport proteins (e.g., ferredoxin) reduce metronidazole → nitroso radicals → DNA strand breakage and synthesis inhibition in anaerobes/protozoa.
Absorption
Well absorbed orally; tₘₐₓ ~1–2 h; oral and IV exposures are comparable (dose-proportional).
Volume of distribution
~0.51–1.1 L/kg; penetrates CSF, saliva, breast milk; therapeutic concentrations in abscess pus.
Protein binding
<20% (low).
Metabolism
Hepatic oxidation (e.g., 2-hydroxymethyl metabolite) and glucuronidation; parent and hydroxyl-metabolite are microbiologically active.
Route of elimination
Primarily urinary (≈60–80% of dose as metabolites and ~20% unchanged); fecal ≈6–15%.
Half-life
~8 h (adults with normal organ function).
Clearance
Renal clearance ≈10 mL/min/1.73 m²; hemodialysis removes ~40–65% of a dose; exposure ↑ with hepatic impairment (dose reduction in Child-Pugh C).
Adverse Effects
Nausea, metallic taste, headache, dizziness; peripheral neuropathy with prolonged/high-dose use; dark urine; rare seizures/encephalopathy.
Toxicity
Overdose: supportive care; monitor neurologic status and hepatic function.
Pathways
Anaerobic nitroreduction → DNA damage; hepatic metabolic clearance; renal elimination of parent + conjugates.
Pharmacogenomic Effects/ADRs
No required PGx; variability in redox/activation pathways can influence susceptibility in pathogens rather than host.
3. Interactions
Drug Interactions
Warfarin/anticoagulants (↑ INR; monitor), busulfan (↑ exposure; avoid unless essential), enzyme modulators—cimetidine (↑ levels), phenytoin/phenobarbital (↓ levels), lithium (toxicity risk; monitor). Additive QT-prolongation risk with other QT-prolonging drugs reported in labeling.
Food Interactions
Food has minimal PK impact. Alcohol: avoid during therapy and for ≥3 days after last dose.
4. Categories
ATC Codes
J01XD01 (systemic imidazole antibacterial); A01AB17 (oral topical, stomatological); D06BX01 (dermatological, topical); G01AF01 (gynecological); P01AB01 (antiprotozoal, nitroimidazole)
Drug Categories
Antibacterial; Antiprotozoal; Nitroimidazole; Small molecule
Chemical Taxonomy
5-Nitroimidazole core with 2-hydroxyethyl side chain; weakly basic heteroaromatic nitro compound
Affected organisms
Humans (therapeutic use); targets anaerobic bacteria and select protozoa
5. Chemical Identifiers
UNII
140QMO216E
CAS number
443-48-1
InChI Key
VAOCPAMSLUNLGC-UHFFFAOYSA-N
InChI
InChI=1S/C6H9N3O3/c1-5-7-4-6(9(11)12)8(5)2-3-10/h4,10H,2-3H2,1H3
IUPAC Name
2-(2-methyl-5-nitro-1H-imidazol-1-yl)ethanol
SMILES
OCCn1c(C)ncc1N+[O-]
6. References
PubChem Compound Summary: Metronidazole — formula C₆H₉N₃O₃, MW ~171.15, identifiers (CAS, InChI/Key, SMILES). pubchem.ncbi.nlm.nih.gov
FDA GSRS/UNII — UNII 140QMO216E, InChIKey VAOCPAMSLUNLGC-UHFFFAOYSA-N. precision.fda.gov
DailyMed/FDA labeling — absorption (well absorbed; tₘₐₓ 1–2 h), protein binding <20%, distribution to CSF/saliva/milk, urinary excretion 60–80% (≈20% unchanged), half-life ~8 h, renal clearance ~10 mL/min/1.73 m², hemodialysis removes 40–65%, hepatic-impairment dose guidance, mechanism text. DailyMed+1
Clinical PK reviews — Vd ~0.51–1.1 L/kg; low protein binding. PubMed+1
KEGG DRUG — ATC set J01XD01, A01AB17, D06BX01, G01AF01, P01AB01; formula and mass. KEGG
AccessData FDA labels and product labeling excerpts — distribution note, mechanism phrasing, interaction cautions (busulfan, QT risk, enzyme modulators). FDA Access Data+2Pfizer Labeling+2

I am pursuing MBA in pharmaceutical management from NIPER Hyderabad with a strong academic record and proven success in national-level pharmacy entrance exams. I secured AIR 61 in NIPER 2024 (MS/M.Pharm) and AIR 27 in NIPER MBA, along with AIR 147 in GPAT 2024 and AIR 907 in GPAT 2023. I also achieved AIR 6 in AIIMS CRE-2025 for Drug Store Keeper and was selected as a Pharmacist (AIR 61) for ESIC. Additionally, I was the Runner-Up in Round 2 of the EY Case Study Competition.
At PharmacyFreak.com, I aim to guide future pharmacists through expert content, exam strategies, and insightful resources based on real experience and academic excellence.
Mail- harsh@pharmacyfreak.com