Table of Contents
Mechanism of Action of Miralax
Introduction
Miralax, with the active ingredient polyethylene glycol 3350 (PEG 3350), is a commonly used osmotic laxative indicated for the treatment of occasional constipation. Its popularity stems from its non-stimulant mechanism, gentle action on the gastrointestinal tract, and favorable safety profile. Miralax does not cause dependency and is generally well-tolerated, making it a first-line option for bowel regulation in adults and adolescents.
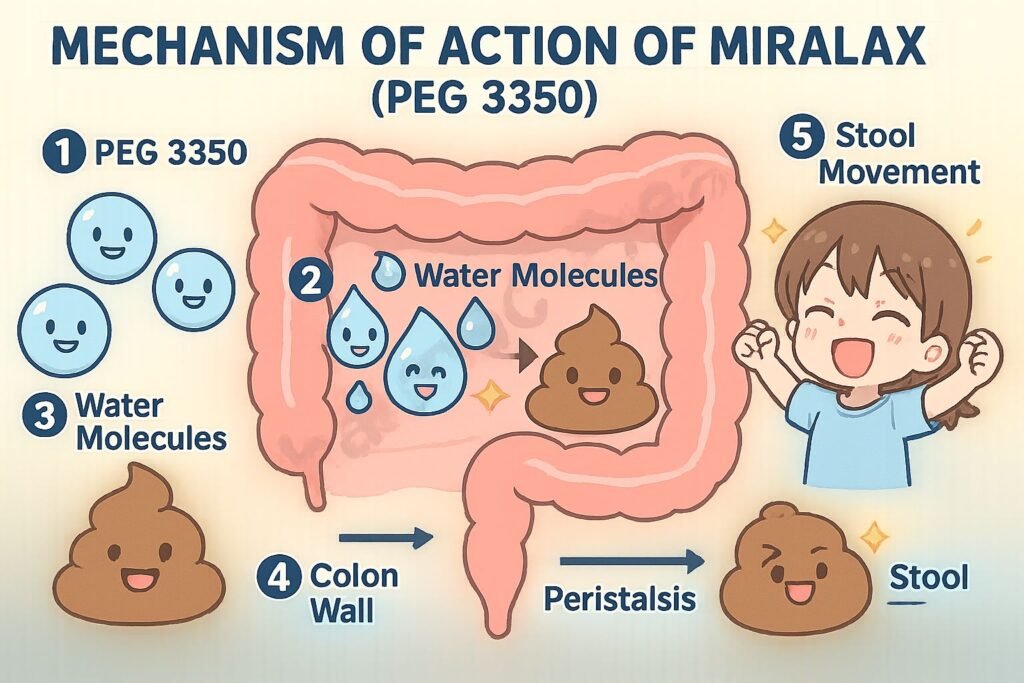
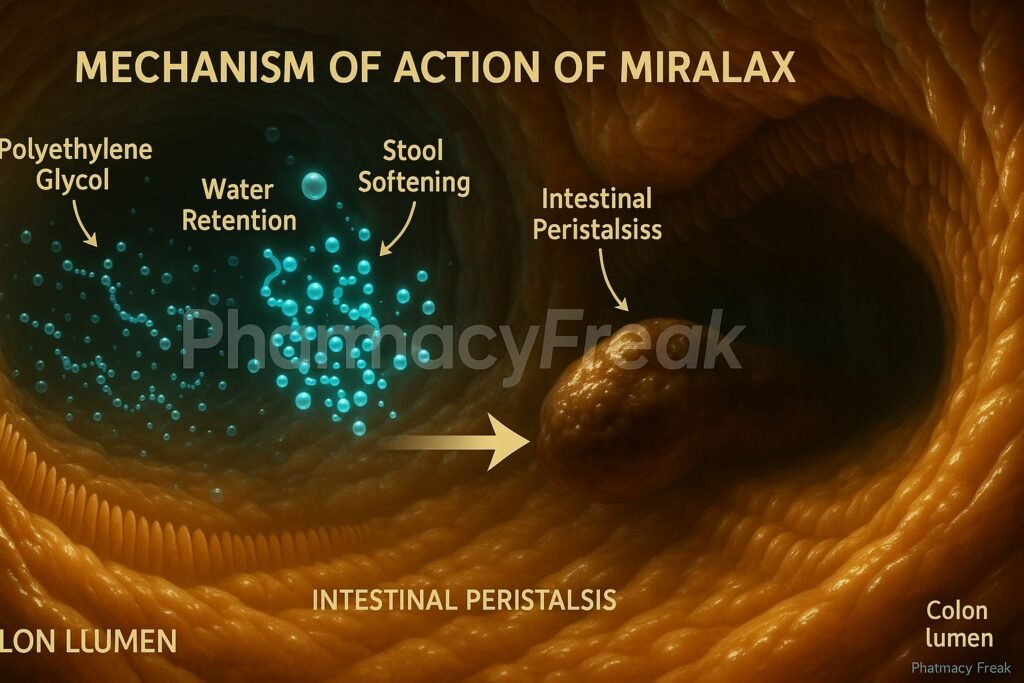
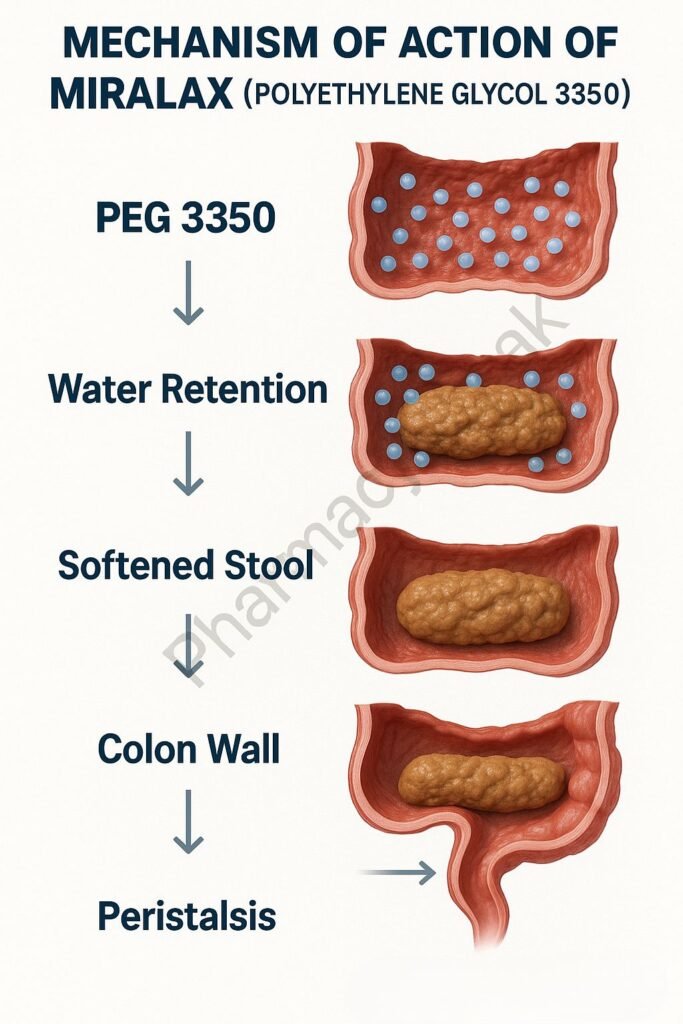
Mechanism of Action (Step-wise)
1. Osmotic Water Retention
PEG 3350 is an inert, non-absorbable compound that retains water in the intestinal lumen by osmosis.
2. Increased Stool Hydration
The retained water softens stool consistency, enhancing its bulk and promoting easier passage.
3. Enhanced Colonic Motility
Increased stool volume stretches the colonic walls, stimulating gentle peristalsis.
4. Lack of Fermentation
Unlike fiber-based laxatives, PEG 3350 is not fermented by gut bacteria, reducing gas and bloating.
5. Absence of Systemic Absorption
PEG remains confined to the GI tract, minimizing systemic exposure and associated risks.
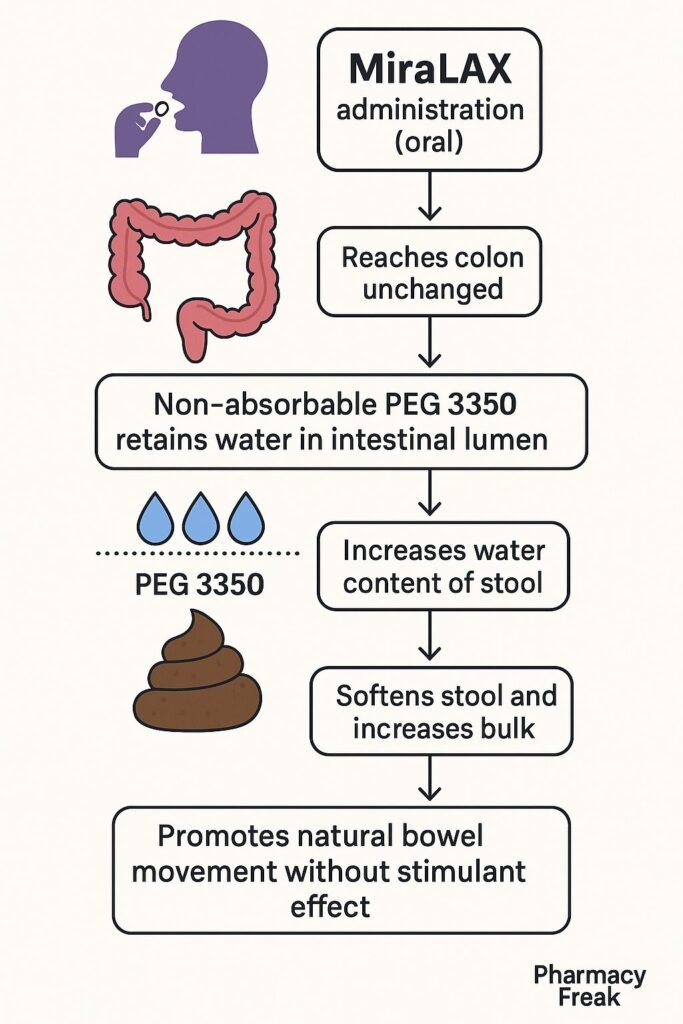
Pharmacokinetics
- Route of Administration: Oral
- Absorption: Negligible systemic absorption
- Onset of Action: Typically 1 to 3 days
- Metabolism: Not metabolized
- Excretion: Eliminated unchanged in feces
Clinical Uses
- Occasional constipation in adults and adolescents (17+ years)
- Short-term bowel regulation
- Off-label use in pediatric constipation under medical supervision
- Often used in bowel preparation regimens in combination with electrolytes
Adverse Effects
- Common: Bloating, flatulence, abdominal discomfort, diarrhea
- Rare: Nausea, cramping
- Pediatric Concerns: Though used off-label, there have been unconfirmed reports of behavioral changes in children
- Precautions: Prolonged use may lead to electrolyte imbalance if fluid intake is inadequate
Comparative Analysis
| Parameter | Miralax (PEG 3350) | Stimulant Laxatives | Fiber Laxatives |
|---|---|---|---|
| Mechanism | Osmotic water retention | Bowel wall stimulation | Bulk-forming |
| Onset | 1–3 days | 6–12 hours | 12–72 hours |
| Gas/Bloating | Minimal | Moderate | Often |
| Risk of Dependence | None | Possible with long use | None |
| Common Use | Chronic constipation | Acute constipation | Mild constipation |
Miralax offers a non-irritating, gradual relief approach to constipation, contrasting with the rapid yet harsher effects of stimulant laxatives.
Multiple Choice Questions (MCQs)
1. What is the primary mechanism of action of Miralax?
a) Inhibition of sodium channels
b) Increase in serotonin release
c) Osmotic water retention in colon
d) Direct stimulation of peristalsis
Answer: c) Osmotic water retention in colon
2. What is the active ingredient in Miralax?
a) Lactulose
b) Polyethylene glycol 3350
c) Docusate sodium
d) Bisacodyl
Answer: b) Polyethylene glycol 3350
3. Which of the following is a common side effect of Miralax?
a) Bradycardia
b) Headache
c) Bloating
d) Hypoglycemia
Answer: c) Bloating
4. How is Miralax eliminated from the body?
a) Renal excretion
b) Hepatic metabolism
c) Exhalation
d) Fecal excretion
Answer: d) Fecal excretion
5. How long does Miralax usually take to work?
a) 1–3 hours
b) 6–12 hours
c) 1–3 days
d) 4–7 days
Answer: c) 1–3 days
FAQs
Q1. Can Miralax be used daily?
Yes, but it should be under medical supervision if used beyond 7 days to avoid potential electrolyte imbalance.
Q2. Is Miralax safe for children?
Though not FDA-approved for those under 17, it is frequently used off-label in pediatric constipation with appropriate dosing.
Q3. Does Miralax cause dependence?
No, it works by osmosis and does not stimulate bowel nerves, minimizing risk of dependency.
Q4. Can Miralax be mixed with other medications?
It should be taken separately from other medications by at least 1–2 hours to avoid interference with absorption.
Q5. Is Miralax suitable for bowel prep before procedures?
Yes, but usually in combination with electrolyte solutions under medical guidance.
References
- Goodman & Gilman’s The Pharmacological Basis of Therapeutics, 12th Edition
- KD Tripathi, Essentials of Medical Pharmacology, 7th Edition
- Standard Clinical Guidelines for Constipation Management
- Product Monograph for Polyethylene Glycol 3350
- Clinical Experience Reports in Gastroenterology
Related Internal Links

I am pursuing MBA in pharmaceutical management from NIPER Hyderabad with a strong academic record and proven success in national-level pharmacy entrance exams. I secured AIR 61 in NIPER 2024 (MS/M.Pharm) and AIR 27 in NIPER MBA, along with AIR 147 in GPAT 2024 and AIR 907 in GPAT 2023. I also achieved AIR 6 in AIIMS CRE-2025 for Drug Store Keeper and was selected as a Pharmacist (AIR 61) for ESIC. Additionally, I was the Runner-Up in Round 2 of the EY Case Study Competition.
At PharmacyFreak.com, I aim to guide future pharmacists through expert content, exam strategies, and insightful resources based on real experience and academic excellence.
Mail- harsh@pharmacyfreak.com

