Table of Contents
Introduction
Lubiprostone is a chloride channel activator used primarily to manage chronic idiopathic constipation, irritable bowel syndrome with constipation (IBS-C), and opioid-induced constipation. Unlike traditional laxatives, lubiprostone targets specific ion channels on the gastrointestinal epithelium to enhance fluid secretion and promote intestinal motility. This unique mechanism ensures more physiologic bowel movements with minimal systemic absorption.
Mechanism of Action (Step-wise)
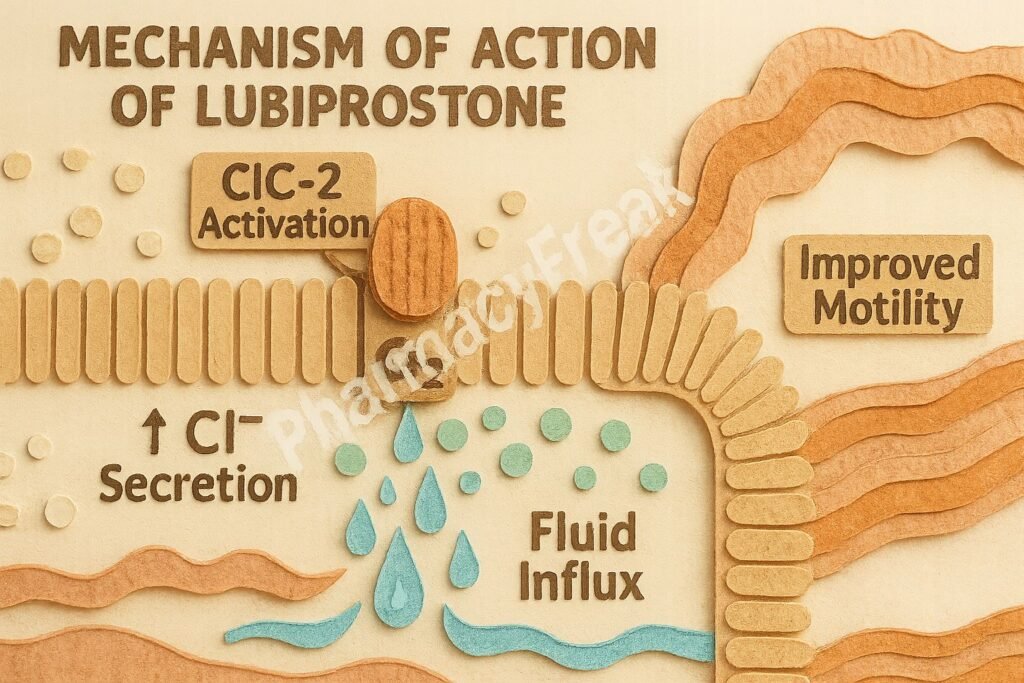
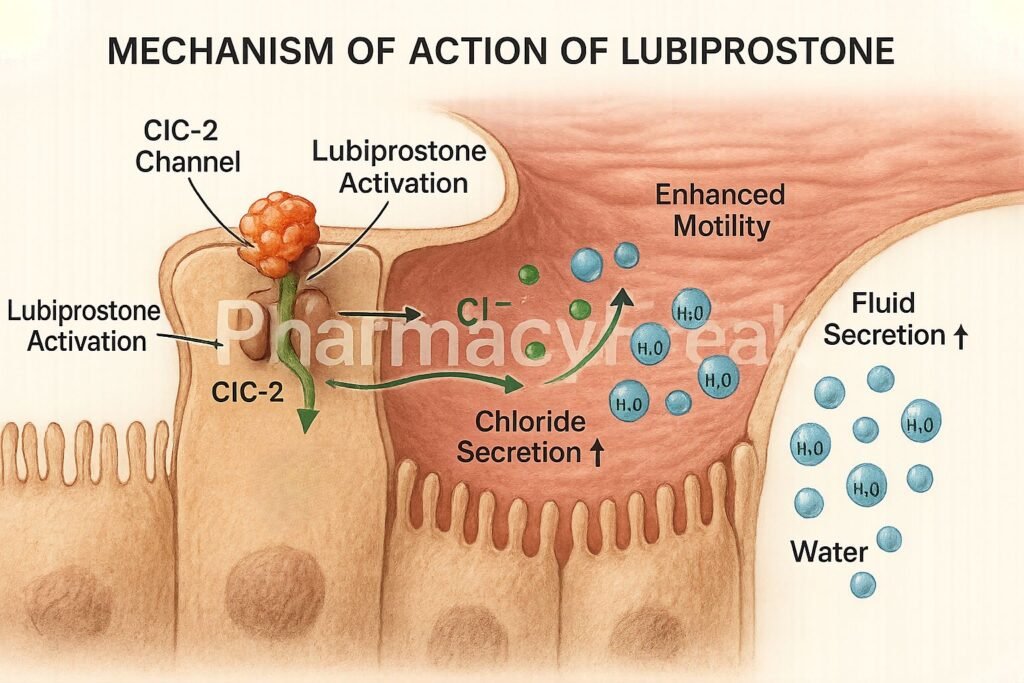
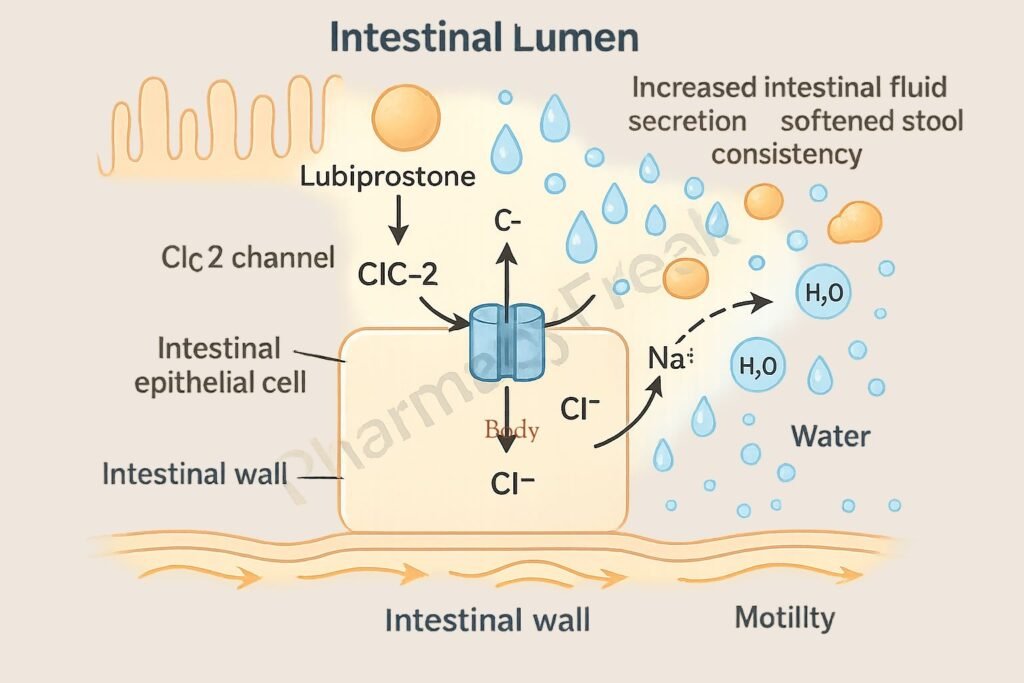
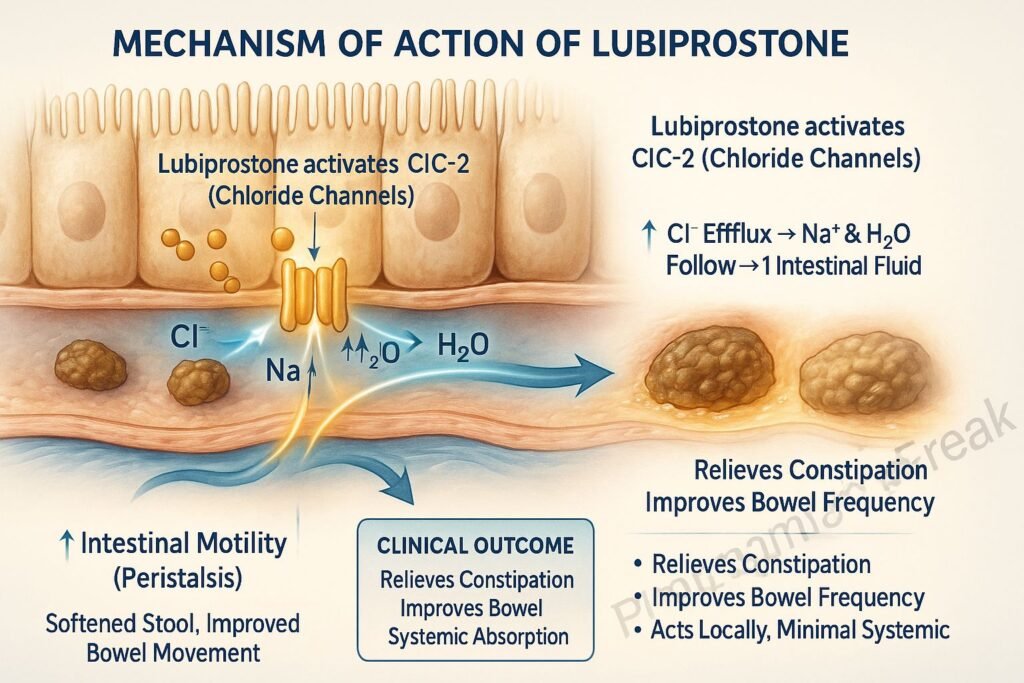
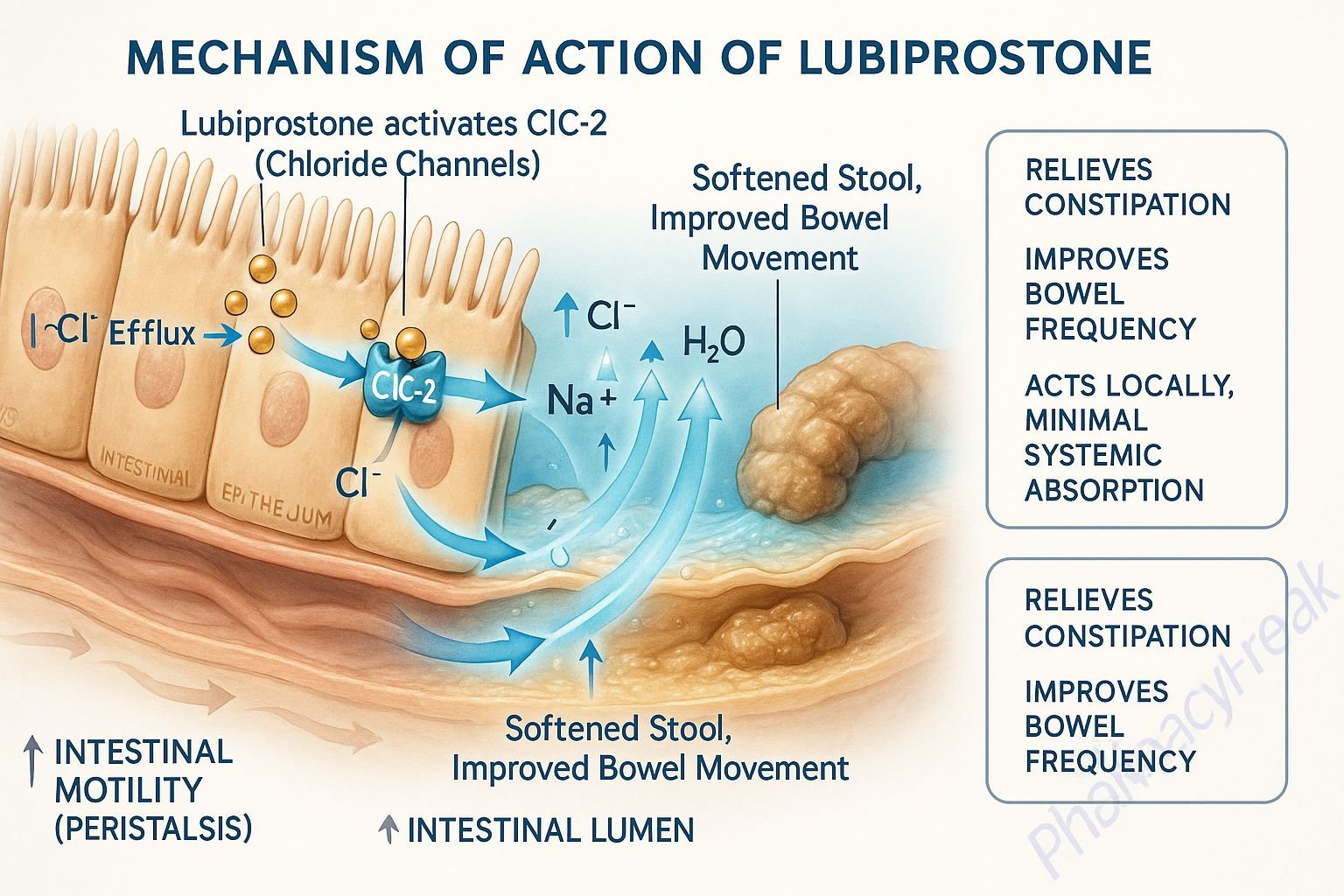
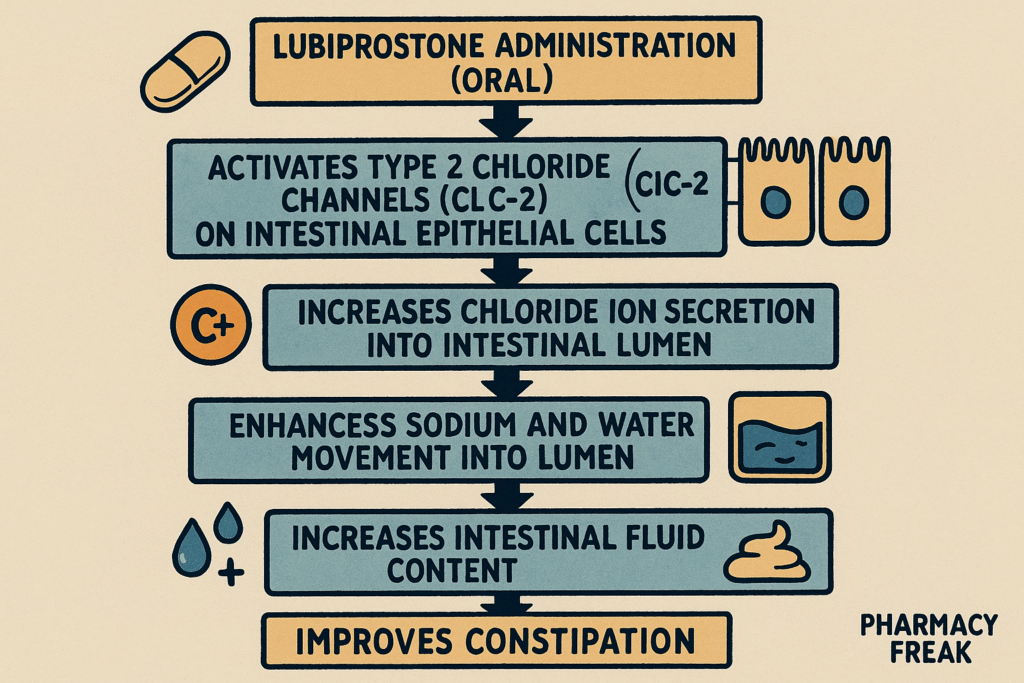
1. Activation of ClC-2 Chloride Channels
Lubiprostone specifically activates type 2 chloride channels (ClC-2) located on the apical side of gastrointestinal epithelial cells.
2. Enhanced Chloride Ion Efflux
This activation leads to an increased efflux of chloride ions into the intestinal lumen.
3. Passive Sodium and Water Secretion
The movement of chloride ions is followed by passive sodium and water secretion, increasing luminal fluid content.
4. Softening of Stool
The enhanced luminal hydration softens stools and increases their volume.
5. Stimulation of Intestinal Transit
The net result is improved intestinal motility and facilitation of stool passage.
Pharmacokinetics
- Route of Administration: Oral
- Bioavailability: Low systemic availability (minimal absorption)
- Onset of Action: Within 24–48 hours
- Half-Life: Not well-defined due to local action
- Metabolism: Rapidly metabolized in the stomach and jejunum
- Excretion: Fecal (as inactive metabolites)
Clinical Uses
- Chronic idiopathic constipation (CIC)
- Irritable bowel syndrome with constipation (IBS-C) in adult women
- Opioid-induced constipation (OIC) in chronic non-cancer pain patients
Adverse Effects
- Nausea (most common)
- Diarrhea
- Abdominal pain and distension
- Flatulence
- Headache
- Dyspnea (transient, reported shortly after dosing)
- Contraindications: Mechanical gastrointestinal obstruction, severe diarrhea
Comparative Analysis
| Parameter | Lubiprostone | Linaclotide |
|---|---|---|
| Mechanism | ClC-2 chloride channel activator | Guanylate cyclase-C agonist |
| Primary Use | CIC, IBS-C, OIC | CIC, IBS-C |
| Onset of Action | 24–48 hours | ~24 hours |
| Systemic Absorption | Minimal | Minimal |
| Major Side Effect | Nausea | Diarrhea |
Multiple Choice Questions (MCQs)
1. Lubiprostone acts by activating which type of channel?
a) Potassium channel
b) ClC-2 chloride channel
c) Sodium channel
d) Calcium channel
Answer: b) ClC-2 chloride channel
2. The most common adverse effect of lubiprostone is:
a) Headache
b) Diarrhea
c) Nausea
d) Constipation
Answer: c) Nausea
3. Lubiprostone is primarily used for:
a) Ulcerative colitis
b) Chronic idiopathic constipation
c) Infectious diarrhea
d) Hepatic encephalopathy
Answer: b) Chronic idiopathic constipation
4. How does lubiprostone promote water secretion into the intestines?
a) By stimulating aldosterone
b) By inhibiting sodium reabsorption
c) Through chloride ion efflux
d) By activating aquaporins
Answer: c) Through chloride ion efflux
5. What is a contraindication for lubiprostone use?
a) Diabetes mellitus
b) IBS-D
c) Mechanical gastrointestinal obstruction
d) Hypertension
Answer: c) Mechanical gastrointestinal obstruction
FAQs
Q1. Is lubiprostone absorbed systemically?
No, it has minimal systemic absorption and works locally in the GI tract.
Q2. Can lubiprostone be used in pregnancy?
It is classified as pregnancy category C; use only if clearly needed.
Q3. How quickly does lubiprostone work?
Relief of constipation symptoms usually begins within 1–2 days.
Q4. Is lubiprostone effective in opioid-induced constipation?
Yes, it is approved for OIC in adults with chronic non-cancer pain.
Q5. Can lubiprostone be crushed or chewed?
No, the capsules should be swallowed whole with food and water.
References
- Goodman & Gilman’s The Pharmacological Basis of Therapeutics, 12th Edition
- KD Tripathi, Essentials of Medical Pharmacology, 7th Edition
- FDA Prescribing Information for Lubiprostone
- Clinical guidelines on constipation management
Related Internal Links

I am pursuing MBA in pharmaceutical management from NIPER Hyderabad with a strong academic record and proven success in national-level pharmacy entrance exams. I secured AIR 61 in NIPER 2024 (MS/M.Pharm) and AIR 27 in NIPER MBA, along with AIR 147 in GPAT 2024 and AIR 907 in GPAT 2023. I also achieved AIR 6 in AIIMS CRE-2025 for Drug Store Keeper and was selected as a Pharmacist (AIR 61) for ESIC. Additionally, I was the Runner-Up in Round 2 of the EY Case Study Competition.
At PharmacyFreak.com, I aim to guide future pharmacists through expert content, exam strategies, and insightful resources based on real experience and academic excellence.
Mail- harsh@pharmacyfreak.com