Table of Contents
Introduction
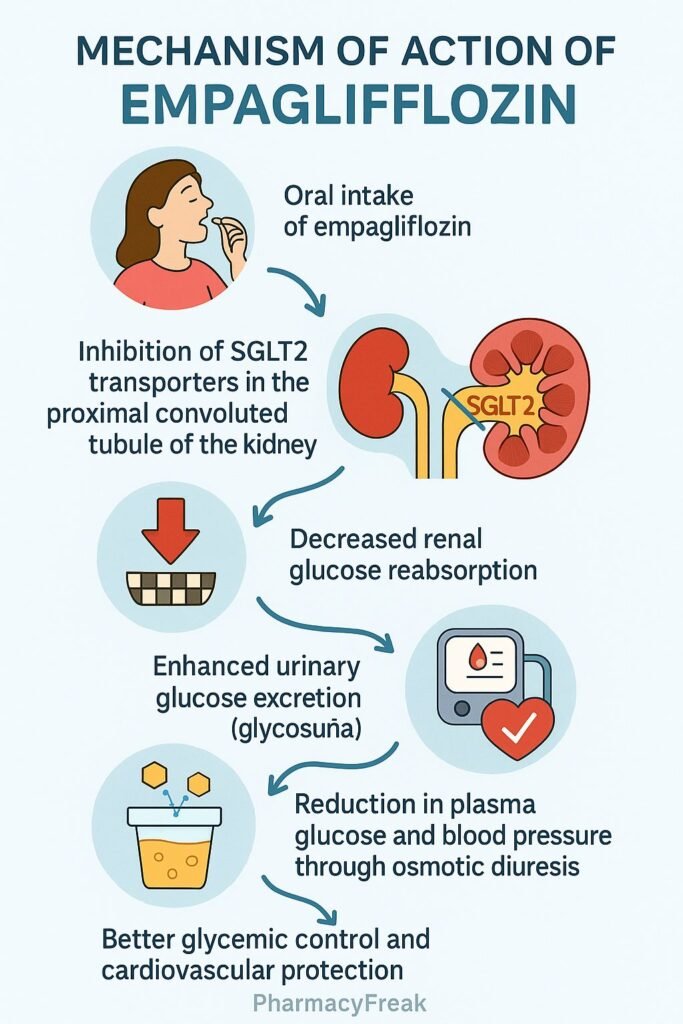
Empagliflozin is an oral sodium–glucose co-transporter 2 (SGLT2) inhibitor that lowers blood glucose by promoting urinary glucose excretion. It also provides cardiovascular and renal protective benefits, and is approved for type 2 diabetes mellitus, heart failure, and chronic kidney disease.
Step-by-Step Mechanism of Action
- Selective SGLT2 Inhibition
Empagliflozin selectively blocks SGLT2 transporters in the proximal renal tubules, responsible for ~90% of glucose reabsorption. - Enhanced Glucosuria
By inhibiting glucose reabsorption, it increases urinary glucose excretion, lowering plasma glucose and HbA1c. - Osmotic Diuresis & Natriuresis
Increased glucose in urine promotes osmotic diuresis and sodium loss, reducing plasma volume and lowering blood pressure. - Weight Reduction
Caloric loss from glucose excretion contributes to modest weight loss. - Cardiorenal Benefits
Volume reduction, improved hemodynamics, reduced intraglomerular pressure, and metabolic effects enhance heart failure outcomes and slow kidney disease progression.
Pharmacokinetic Parameters
| Parameter | Value |
|---|---|
| Route | Oral, taken once daily |
| Bioavailability | ~78% |
| Protein Binding | ~86% |
| Metabolism | Glucuronidation via UGT enzymes |
| Half-life | ~12 hours |
| Excretion | Approximately 54% as metabolites in urine; remainder in feces |
Clinical Uses
- Glycemic control in type 2 diabetes mellitus
- Reducing risk of cardiovascular death in type 2 diabetes and established CV disease
- Treatment of heart failure with reduced ejection fraction
- Management of chronic kidney disease with or without diabetes
Adverse Effects
- Genital mycotic infections
- Urinary tract infections
- Volume depletion symptoms (e.g., dizziness, hypotension)
- Rare cases of euglycemic diabetic ketoacidosis
- Slight rise in LDL cholesterol; rare cases of Fournier’s gangrene
Comparative Analysis
| Agent | SGLT2 Selectivity | Unique Benefits |
|---|---|---|
| Empagliflozin | High | Strong CV mortality reduction |
| Dapagliflozin | High | CKD protection unique approval |
| Canagliflozin | Moderate | Modest SGLT1 effect; amputation risk |
MCQs
- Empagliflozin inhibits which transporter?
a) SGLT1 b) SGLT2 c) GLUT4 d) Na⁺/K⁺ ATPase
Answer: b) SGLT2 - Its main glucose-lowering effect is via:
a) Insulin release b) Hepatic gluconeogenesis suppression c) Urinary glucose excretion d) Intestinal absorption
Answer: c) Urinary glucose excretion - Another beneficial effect is:
a) Hyperlipidemia b) Weight gain c) Weight loss d) CNS stimulation
Answer: c) Weight loss - Osmotic diuresis leads to:
a) Volume expansion b) Natriuresis and BP reduction c) Hypernatremia d) Hypercalcemia
Answer: b) Natriuresis and BP reduction - A rare but serious adverse event is:
a) Hypoglycemia b) Euglycemic ketoacidosis c) Pancreatitis d) Hypothyroidism
Answer: b) Euglycemic ketoacidosis - Empagliflozin has half-life of approximately:
a) 4 hours b) 8 hours c) 12 hours d) 24 hours
Answer: c) 12 hours - Its cardiovascular benefit mainly includes:
a) Stroke prevention b) Reducing CV death c) Preventing arrhythmias d) Improving ejection fraction
Answer: b) Reducing CV death - Common genitourinary side effect:
a) Bacterial vaginosis b) Genital mycoses c) Hepatitis d) Alopecia
Answer: b) Genital mycoses - Empagliflozin is metabolized primarily via:
a) CYP3A4 b) Glucuronidation c) Renal unchanged d) Hydrolysis
Answer: b) Glucuronidation - Use is contraindicated in:
a) Heart failure b) Chronic kidney disease c) Type 1 diabetes d) Obesity without diabetes
Answer: c) Type 1 diabetes
FAQs
1. Does empagliflozin cause hypoglycemia?
No, unless used alongside insulin or sulfonylureas.
2. Are doses adjusted for renal impairment?
Yes—efficacy decreases as eGFR drops; check local guidelines.
3. How soon do cardiovascular benefits appear?
Benefits typically emerge within weeks to a few months.
4. Can it be used in non-diabetic CKD?
Yes, empagliflozin is approved for CKD irrespective of diabetes status.
5. How can genital infections be prevented?
Maintain good hygiene and consider prophylactic measures if recurrent.
References
- PubMed: Cardiovascular and renal outcomes with empagliflozin
- DrugBank: Empagliflozin pharmacokinetics
- StatPearls: SGLT2 inhibitors in heart failure
- FDA Label: Empagliflozin clinical data
- PMC article: Mechanisms of SGLT2 inhibitors

I am pursuing MBA in pharmaceutical management from NIPER Hyderabad with a strong academic record and proven success in national-level pharmacy entrance exams. I secured AIR 61 in NIPER 2024 (MS/M.Pharm) and AIR 27 in NIPER MBA, along with AIR 147 in GPAT 2024 and AIR 907 in GPAT 2023. I also achieved AIR 6 in AIIMS CRE-2025 for Drug Store Keeper and was selected as a Pharmacist (AIR 61) for ESIC. Additionally, I was the Runner-Up in Round 2 of the EY Case Study Competition.
At PharmacyFreak.com, I aim to guide future pharmacists through expert content, exam strategies, and insightful resources based on real experience and academic excellence.
Mail- harsh@pharmacyfreak.com

