Table of Contents
Introduction
Cytarabine (also known as cytosine arabinoside or Ara-C) is a pyrimidine antimetabolite chemotherapeutic agent primarily used in the treatment of acute leukemias, especially acute myelogenous leukemia (AML). It is a cell cycle–specific drug, acting predominantly during the S-phase of the cell cycle. Cytarabine is a cornerstone drug in hematologic malignancies and is a high-yield topic in pharmacology, oncology, and hematology examinations due to its unique intracellular activation and DNA synthesis inhibition.
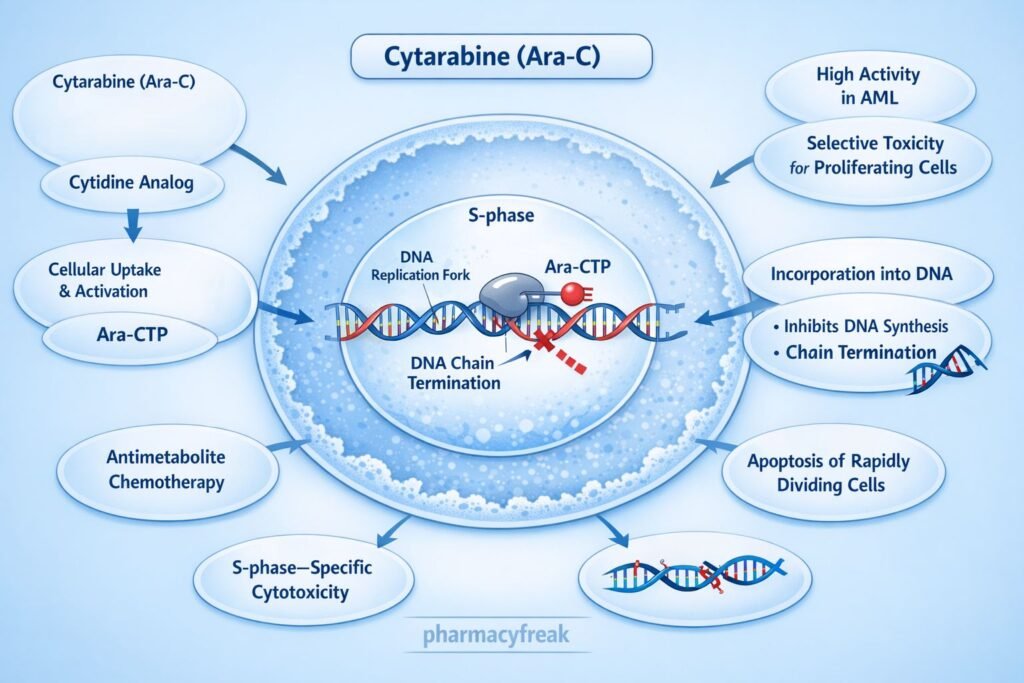
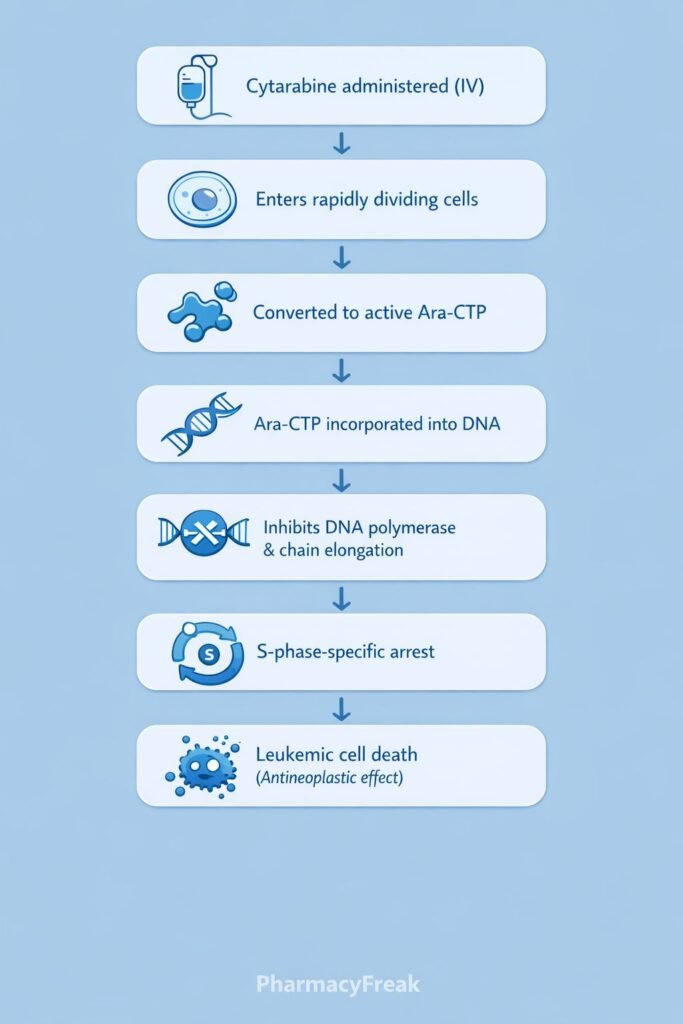
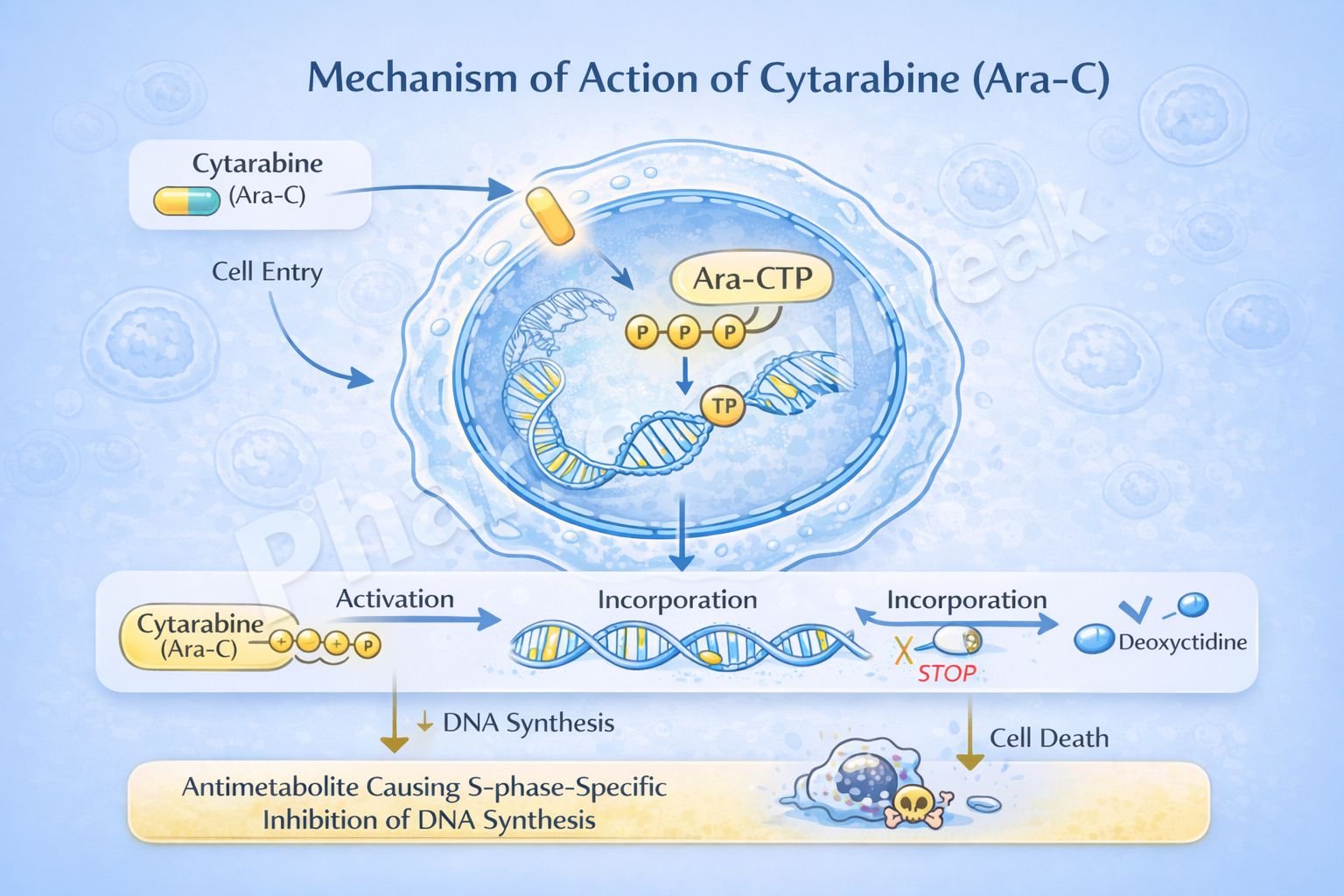
Mechanism of Action (Step-wise)
Cytarabine inhibits DNA synthesis by acting as a false nucleotide after intracellular activation.
Step-wise mechanism:
- Cellular Uptake
Cytarabine enters rapidly dividing cells via nucleoside transporters. - Intracellular Activation
Inside the cell, cytarabine is phosphorylated by deoxycytidine kinase to form:- Cytarabine monophosphate (Ara-CMP)
- Cytarabine diphosphate (Ara-CDP)
- Cytarabine triphosphate (Ara-CTP) – the active metabolite
- Competition with Deoxycytidine Triphosphate
Ara-CTP competes with deoxycytidine triphosphate for incorporation into DNA. - Incorporation into DNA
Ara-CTP is incorporated into the growing DNA strand during replication. - Inhibition of DNA Polymerase
Incorporation of Ara-CTP inhibits DNA polymerase activity. - Premature Chain Termination
Abnormal sugar moiety (arabinose) prevents proper elongation of the DNA strand. - S-Phase–Specific Cytotoxicity
DNA synthesis is halted, leading to apoptosis of rapidly dividing leukemic cells.
Pharmacokinetics
- Administration: Intravenous, subcutaneous, intrathecal
- Absorption: Not effective orally due to rapid deamination
- Distribution: Widely distributed; penetrates CNS when given intrathecally
- Metabolism: Rapid hepatic deamination to inactive uracil arabinoside
- Elimination: Renal excretion
- Half-life: Short (10–20 minutes)
- Special feature: Requires continuous infusion or frequent dosing
Clinical Uses
Cytarabine is primarily used in hematologic malignancies:
- Acute myelogenous leukemia (AML) – first-line therapy
- Acute lymphoblastic leukemia (ALL)
- Chronic myelogenous leukemia (blast crisis)
- Non-Hodgkin lymphoma (selected regimens)
- Intrathecal use for leukemic meningitis
High-dose cytarabine is used in consolidation therapy for AML.
Adverse Effects
Cytarabine toxicity primarily affects rapidly dividing tissues:
- Hematologic:
- Severe myelosuppression
- Leukopenia
- Thrombocytopenia
- Gastrointestinal:
- Nausea
- Vomiting
- Mucositis
- Neurologic (high-dose):
- Cerebellar toxicity (ataxia, dysarthria)
- Ocular:
- Chemical conjunctivitis
- Cytarabine syndrome:
- Fever
- Myalgia
- Bone pain
- Rash
Comparative Analysis (must include a table + explanation)
Comparison of Antimetabolite Chemotherapy Drugs
| Feature | Cytarabine | Methotrexate | 5-Fluorouracil |
|---|---|---|---|
| Drug class | Pyrimidine analog | Folic acid analog | Pyrimidine analog |
| Cell cycle specificity | S-phase | S-phase | S-phase |
| Primary action | DNA polymerase inhibition | DHFR inhibition | Thymidylate synthase inhibition |
| Major use | AML | Leukemia, solid tumors | Solid tumors |
| Unique toxicity | Cerebellar toxicity | Mucositis | Hand–foot syndrome |
Explanation:
Cytarabine differs from other antimetabolites by acting directly as a false nucleotide incorporated into DNA. Its selective activity against rapidly proliferating leukemic cells makes it a mainstay in AML therapy, whereas methotrexate and 5-FU are more commonly used in solid tumors.
MCQs (10–15)
- Cytarabine is classified as a:
a) Alkylating agent
b) Antitumor antibiotic
c) Antimetabolite
d) Mitotic inhibitor
Answer: c) Antimetabolite
- Cytarabine is most active during which phase of the cell cycle?
a) G₀
b) G₁
c) S
d) M
Answer: c) S
- The active metabolite of cytarabine is:
a) Ara-CMP
b) Ara-CDP
c) Ara-CTP
d) Uracil arabinoside
Answer: c) Ara-CTP
- Cytarabine inhibits DNA synthesis by:
a) Cross-linking DNA
b) Blocking topoisomerase II
c) Inhibiting DNA polymerase
d) Inhibiting thymidylate synthase
Answer: c) Inhibiting DNA polymerase
- Cytarabine is primarily used in:
a) Breast cancer
b) Lung cancer
c) Acute myelogenous leukemia
d) Colon cancer
Answer: c) Acute myelogenous leukemia
- Cytarabine must be given parenterally because:
a) Poor absorption
b) Rapid hepatic deamination
c) Renal toxicity
d) High protein binding
Answer: b) Rapid hepatic deamination
- A dose-limiting toxicity of cytarabine is:
a) Nephrotoxicity
b) Myelosuppression
c) Cardiotoxicity
d) Pulmonary fibrosis
Answer: b) Myelosuppression
- High-dose cytarabine is associated with:
a) Ototoxicity
b) Cerebellar toxicity
c) QT prolongation
d) Peripheral neuropathy
Answer: b) Cerebellar toxicity
- Cytarabine resembles which nucleoside?
a) Adenosine
b) Guanosine
c) Cytidine
d) Thymidine
Answer: c) Cytidine
- Cytarabine is ineffective orally because of:
a) First-pass metabolism
b) Low lipid solubility
c) Rapid deamination
d) Poor stability
Answer: c) Rapid deamination
FAQs (minimum 5)
- What is the primary mechanism of cytarabine?
Inhibition of DNA synthesis by incorporation into DNA and inhibition of DNA polymerase. - Is cytarabine cell cycle–specific?
Yes, it is S-phase specific. - Why is cytarabine mainly used in leukemia?
Because leukemic cells divide rapidly and are highly sensitive to DNA synthesis inhibition. - What is cytarabine syndrome?
A flu-like reaction with fever, myalgia, bone pain, and rash. - Why does high-dose cytarabine cause cerebellar toxicity?
Due to accumulation in CNS tissues affecting neuronal function. - Can cytarabine be given intrathecally?
Yes, for treatment of leukemic meningitis.
References
- Goodman & Gilman’s The Pharmacological Basis of Therapeutics
https://accessmedicine.mhmedical.com - Katzung BG. Basic and Clinical Pharmacology
https://accessmedicine.mhmedical.com - Tripathi KD. Essentials of Medical Pharmacology
https://www.jaypeebrothers.com - Harrison’s Principles of Internal Medicine
https://accessmedicine.mhmedical.com

I am pursuing MBA in pharmaceutical management from NIPER Hyderabad with a strong academic record and proven success in national-level pharmacy entrance exams. I secured AIR 61 in NIPER 2024 (MS/M.Pharm) and AIR 27 in NIPER MBA, along with AIR 147 in GPAT 2024 and AIR 907 in GPAT 2023. I also achieved AIR 6 in AIIMS CRE-2025 for Drug Store Keeper and was selected as a Pharmacist (AIR 61) for ESIC. Additionally, I was the Runner-Up in Round 2 of the EY Case Study Competition.
At PharmacyFreak.com, I aim to guide future pharmacists through expert content, exam strategies, and insightful resources based on real experience and academic excellence.
Mail- harsh@pharmacyfreak.com