Table of Contents
Introduction
Alteplase is a recombinant tissue plasminogen activator (rt-PA) used as a thrombolytic agent. It plays a crucial role in the dissolution of fibrin clots and is utilized primarily in acute ischemic stroke, myocardial infarction, and pulmonary embolism. This agent mimics the naturally occurring enzyme tissue plasminogen activator (tPA), facilitating clot breakdown and restoring blood flow.

Mechanism of Action
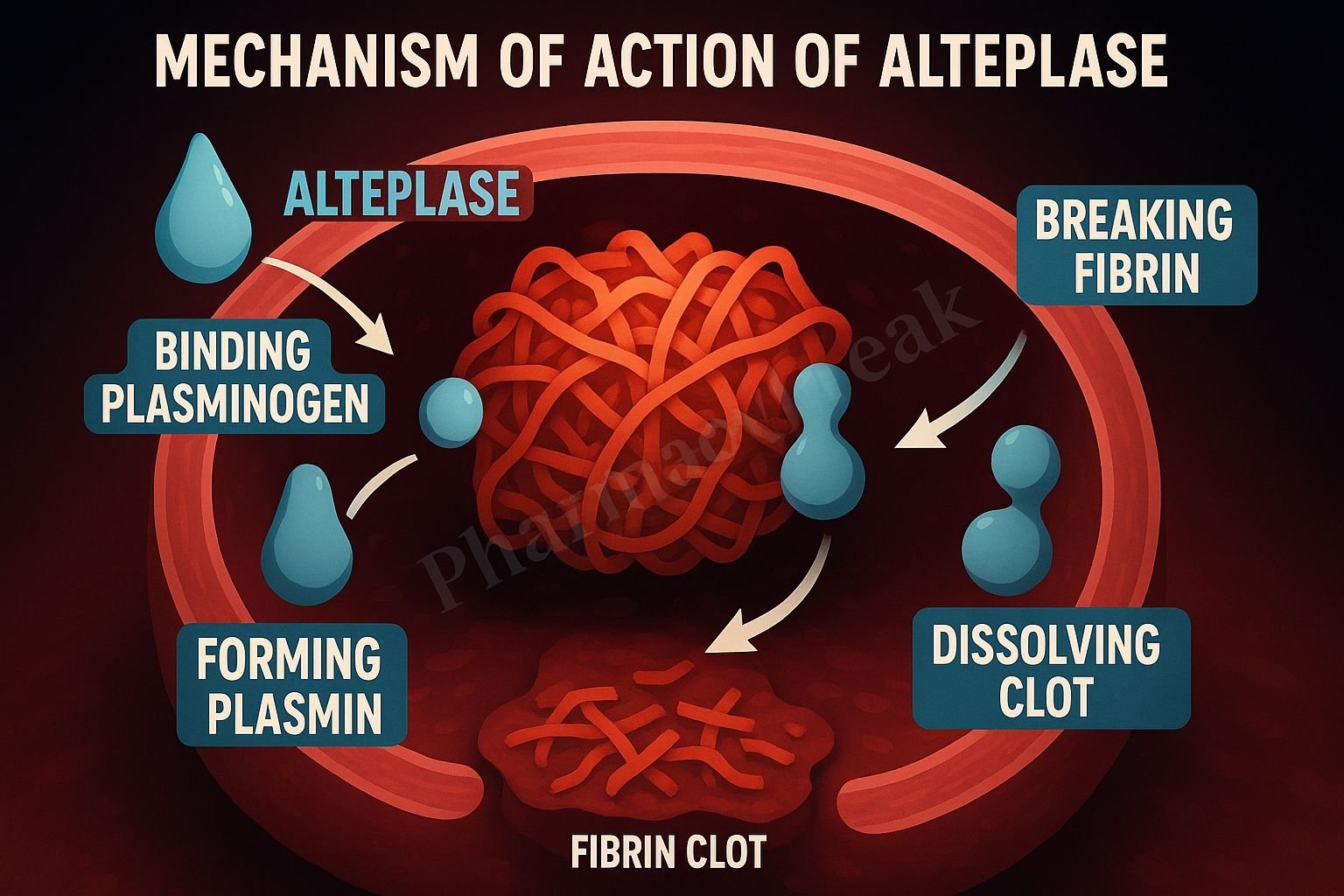
- Binding to Fibrin: Alteplase exhibits a high affinity for fibrin within thrombi.
- Conversion of Plasminogen to Plasmin: Once bound to fibrin, it converts the entrapped plasminogen to plasmin.
- Plasmin-Mediated Fibrinolysis: Plasmin enzymatically degrades the fibrin matrix of the clot.
- Clot Dissolution: The breakdown of fibrin leads to clot lysis and restoration of vessel patency.
- Limited Systemic Action: Alteplase’s action is largely localized to the site of the thrombus due to its fibrin specificity.

Pharmacokinetics
- Absorption: Administered intravenously; immediate systemic availability.
- Distribution: Volume of distribution is low (~0.04 L/kg), indicating limited tissue penetration.
- Metabolism: Rapidly cleared from circulation, primarily via hepatic metabolism.
- Elimination Half-life: Approximately 4–6 minutes (initial), followed by a terminal half-life of ~30–45 minutes.
- Excretion: Metabolites are excreted primarily via the kidneys.
Clinical Uses
- Acute Ischemic Stroke: Administered within 3–4.5 hours of symptom onset.
- Acute Myocardial Infarction: Especially in settings without immediate PCI availability.
- Pulmonary Embolism: In massive PE with hemodynamic instability.
- Catheter Clearance: In small doses to clear occluded central venous catheters.
Adverse Effects
- Hemorrhage: Especially intracranial hemorrhage—most serious risk.
- Allergic Reactions: Rare due to its human recombinant origin.
- Reperfusion Arrhythmias: Can occur during myocardial infarction treatment.
- Nausea, Vomiting, Hypotension: Non-specific systemic reactions.
Comparative Analysis
| Feature | Alteplase | Streptokinase | Tenecteplase |
|---|---|---|---|
| Source | Recombinant human tPA | Bacterial origin | Genetically modified tPA |
| Fibrin specificity | High | Low | Very high |
| Half-life | ~5 min (short) | ~20–30 min | ~20 min |
| Antigenicity | Low | High | Low |
| Cost | High | Low | High |
Explanation: Alteplase is more fibrin-specific than streptokinase, offering localized clot lysis and reducing systemic bleeding risk. Tenecteplase has longer half-life and can be administered as a single bolus.
MCQs
- Alteplase acts primarily by:
- A) Inhibiting thrombin
- B) Converting plasminogen to plasmin
- C) Blocking platelet aggregation
- D) Inhibiting clotting factors
- Alteplase is used in all except:
- A) Ischemic stroke
- B) Myocardial infarction
- C) Hemophilia
- D) Pulmonary embolism
- The half-life of alteplase is approximately:
- A) 20 hours
- B) 2 hours
- C) 5 minutes
- D) 10 minutes
- Alteplase is classified as:
- A) Antiplatelet
- B) Anticoagulant
- C) Fibrinolytic
- D) Thrombin inhibitor
- Which of the following is a major risk with alteplase therapy?
- A) Hyperkalemia
- B) Infection
- C) Intracranial hemorrhage
- D) Renal failure
- Alteplase has high affinity for:
- A) Albumin
- B) Fibrin
- C) Platelets
- D) Thrombin
- What is the primary site of alteplase metabolism?
- A) Kidney
- B) Liver
- C) Lung
- D) Spleen
- Alteplase differs from streptokinase by:
- A) Longer half-life
- B) Higher fibrin specificity
- C) Higher antigenicity
- D) Oral administration
- Alteplase administration should occur within how many hours of stroke symptom onset?
- A) 12 hours
- B) 6 hours
- C) 3–4.5 hours
- D) 24 hours
- Alteplase is a:
- A) Vitamin K antagonist
- B) Factor Xa inhibitor
- C) Recombinant tissue plasminogen activator
- D) Low molecular weight heparin
FAQs
Q1: Is alteplase the same as tPA?
Yes, alteplase is a recombinant form of endogenous tissue plasminogen activator.
Q2: Can alteplase be reversed?
There is no specific reversal agent; management is supportive in case of bleeding.
Q3: How is alteplase administered?
Intravenously, either as a bolus followed by infusion or continuous infusion depending on the indication.
Q4: Is there a risk of allergic reaction with alteplase?
It is minimal due to its human recombinant nature.
Q5: Is alteplase safe during pregnancy?
It is used only when the potential benefit justifies the potential risk.
References
- Goodman & Gilman’s: The Pharmacological Basis of Therapeutics, 12th Edition
- KD Tripathi: Essentials of Medical Pharmacology, 7th Edition
- Harrison’s Principles of Internal Medicine
- Standard Treatment Guidelines by WHO and AHA
Related Internal Links

I am pursuing MBA in pharmaceutical management from NIPER Hyderabad with a strong academic record and proven success in national-level pharmacy entrance exams. I secured AIR 61 in NIPER 2024 (MS/M.Pharm) and AIR 27 in NIPER MBA, along with AIR 147 in GPAT 2024 and AIR 907 in GPAT 2023. I also achieved AIR 6 in AIIMS CRE-2025 for Drug Store Keeper and was selected as a Pharmacist (AIR 61) for ESIC. Additionally, I was the Runner-Up in Round 2 of the EY Case Study Competition.
At PharmacyFreak.com, I aim to guide future pharmacists through expert content, exam strategies, and insightful resources based on real experience and academic excellence.
Mail- harsh@pharmacyfreak.com