Table of Contents
1. Identification
Summary
Aspirin (acetylsalicylic acid) is a nonsteroidal anti-inflammatory drug (NSAID) used for analgesia, antipyresis, anti-inflammation, and—at low doses—irreversible antiplatelet therapy for cardiovascular prevention.
Brand Names
Aspirin, Ecotrin, Aspro, Disprin, Bayer Aspirin (regional variants and generics)
Name
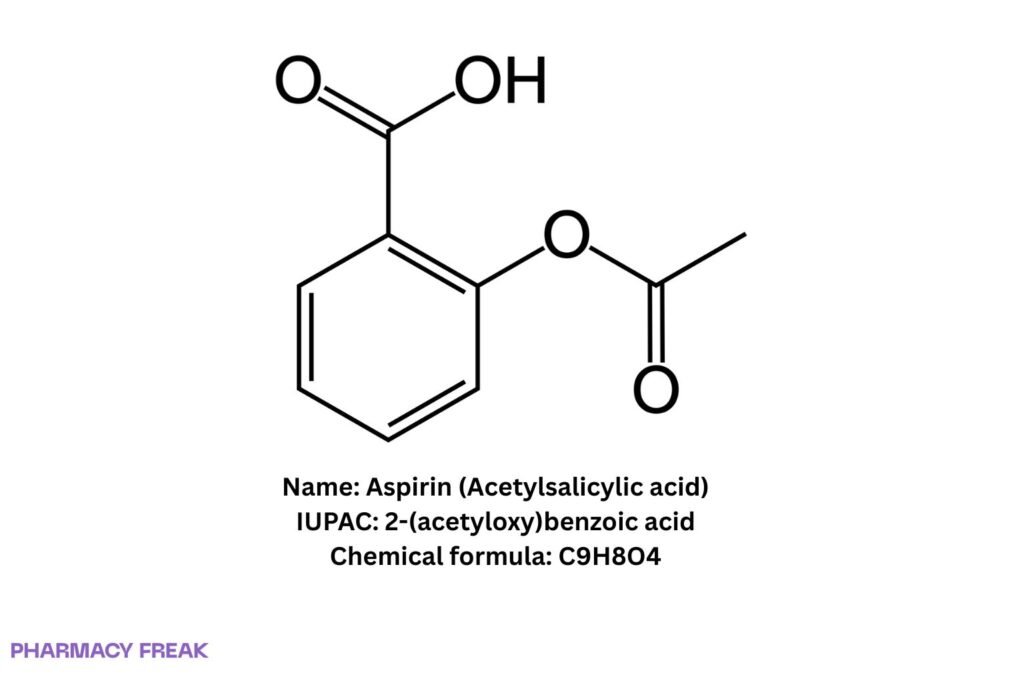
Aspirin (Acetylsalicylic acid)
Background
A salicylate ester developed in the late 19th century; its acetylation of platelet COX-1 underlies long-lasting antiplatelet effects. Widely available as IR/EC tablets, chewables, effervescents, and some combination products.
Modality
Small molecule
Groups
Approved; OTC/Rx (region-dependent)
Structure
Weight
~180.16 g/mol
Chemical Formula
C₉H₈O₄
Synonyms
Acetylsalicylic acid; 2-(acetyloxy)benzoic acid
External IDs
CAS: 50-78-2; PubChem CID: 2244; UNII: R16CO5Y76E; KEGG: D00109; ChEBI: 15365; ChEMBL: CHEMBL25
2. Pharmacology
Indication
Symptomatic treatment of mild–moderate pain and fever; adjunct for inflammatory conditions; low-dose antiplatelet use for secondary prevention of cardiovascular and cerebrovascular events (per guideline/label).
Associated Conditions
Headache, musculoskeletal pain, dysmenorrhoea, fever; chronic coronary disease, prior MI or ischemic stroke/TIA (antiplatelet use as indicated).
Associated Therapies
Often combined with P2Y₁₂ inhibitors in dual antiplatelet therapy (DAPT) when indicated; gastroprotection (e.g., PPI) in high GI-risk patients.
Contraindications & Blackbox Warnings
NSAID class warnings: GI bleeding/ulceration and CV risk (context-dependent). Contraindicated with aspirin/NSAID hypersensitivity, active bleeding, severe uncontrolled peptic ulcer. Reye’s syndrome risk: avoid in children/teens with viral illness. Caution in late pregnancy, bleeding disorders, and severe renal/hepatic impairment.
Pharmacodynamics
Analgesic/antipyretic/anti-inflammatory via prostaglandin reduction; antiplatelet via irreversible suppression of thromboxane A₂ in platelets (effect persists for platelet lifespan).
Mechanism of action
Irreversible acetylation of COX-1/COX-2 decreases prostaglandins and thromboxane. Platelets lack nuclei, so COX-1 activity is permanently reduced until new platelets are formed.
Absorption
Oral absorption is generally rapid; enteric coating or food can delay tₘₐₓ. Aspirin is quickly deacetylated to salicylic acid in plasma/tissues.
Volume of distribution
Approx. ~0.1–0.2 L/kg (salicylate), with pH-dependent tissue distribution.
Protein binding
High (~80–90% for salicylate), albumin-bound; binding decreases at higher concentrations.
Metabolism
Rapid ester hydrolysis to salicylate, followed by hepatic conjugation (salicyluric acid, salicyl phenolic/acyl glucuronides).
Route of elimination
Renal, largely as salicylate conjugates; urinary alkalinization enhances clearance (overdose management principle).
Half-life
Aspirin: short (≈15–20 min). Salicylate: ≈2–3 h at low doses, prolonged (non-linear) at high doses/overdose.
Clearance
Primarily renal for metabolites; rate increases with higher urinary pH.
Adverse Effects
Dyspepsia, nausea, epigastric pain; GI bleeding/ulcer, tinnitus (salicylism), bronchospasm in aspirin-sensitive asthma, rare severe skin reactions.
Toxicity
Overdose (salicylate poisoning) may cause tinnitus, hyperventilation, metabolic acidosis, hyperthermia, altered mental status; management includes activated charcoal (early) and urinary alkalinization/HD when indicated.
Pathways
COX-1/COX-2 acetylation; decreased TXA₂ (platelet) and PGE₂/PGI₂ (tissue).
Pharmacogenomic Effects/ADRs
No routine PGx testing; variability in clinical “aspirin resistance” often relates to adherence, drug interactions, or disease biology rather than a single genetic determinant.
3. Interactions
Drug Interactions
- Anticoagulants/antiplatelets (e.g., warfarin, clopidogrel): ↑ bleeding risk—monitor.
- Other NSAIDs (e.g., ibuprofen): may attenuate aspirin’s antiplatelet effect if taken near the same time; separate dosing per guidance.
- SSRIs/SNRIs, corticosteroids, alcohol: additive GI bleeding risk.
- Uricosurics (probenecid): low-dose aspirin may antagonize uricosuric effect.
- Methotrexate: displacement/clearance effects may ↑ toxicity—use caution.
Food Interactions
Food and enteric coating can delay absorption; alcohol co-use increases GI toxicity risk.
4. Categories
ATC Codes
N02BA01 (analgesic/antipyretic) ; B01AC06 (antithrombotic)
Drug Categories
NSAID; Analgesic; Antipyretic; Antiplatelet; Small molecule
Chemical Taxonomy
Aromatic carboxylic acid ester; salicylate derivative
Affected organisms
Humans (therapeutic use)
5. Chemical Identifiers
UNII
R16CO5Y76E
CAS number
50-78-2
InChI Key
BSYNRYMUTXBXSQ-UHFFFAOYSA-N
InChI
InChI=1S/C9H8O4/c1-6(10)13-8-5-3-2-4-7(8)9(11)12/h2-5H,1H3,(H,11,12)
IUPAC Name
2-(acetyloxy)benzoic acid
SMILES
CC(=O)Oc1ccccc1C(=O)O
6. References
- PubChem Compound Summary: Aspirin (CID 2244) — identifiers, formula, weight, canonical SMILES/InChI.
- FDA/US labeling (DailyMed): Aspirin products — class warnings (GI/CV), pediatric Reye’s caution, dosing/administration.
- StatPearls: Aspirin — pharmacology, PD/PK, toxicity, clinical cautions.
- WHO Model List of Essential Medicines — aspirin listings and indications context.
- ATC/DDD Index — N02BA01 and B01AC06 classifications, DDD.
- NIST/InChI Trust resources — InChI and InChIKey confirmation.
- Review guidance on ibuprofen–aspirin timing for antiplatelet effect preservation (regulatory safety communications and label Q&A).

I am pursuing MBA in pharmaceutical management from NIPER Hyderabad with a strong academic record and proven success in national-level pharmacy entrance exams. I secured AIR 61 in NIPER 2024 (MS/M.Pharm) and AIR 27 in NIPER MBA, along with AIR 147 in GPAT 2024 and AIR 907 in GPAT 2023. I also achieved AIR 6 in AIIMS CRE-2025 for Drug Store Keeper and was selected as a Pharmacist (AIR 61) for ESIC. Additionally, I was the Runner-Up in Round 2 of the EY Case Study Competition.
At PharmacyFreak.com, I aim to guide future pharmacists through expert content, exam strategies, and insightful resources based on real experience and academic excellence.
Mail- harsh@pharmacyfreak.com