Table of Contents
ASEPTIC AREA AND MICROBIAL CONTROL
Aseptic techniques are used to prevent microbial and particular contamination into ophthalmic and parenteral products. Control of microbial contamination is also necessary to eliminate toxic bacterial products. Terminally sterilized products (products sealed in a container and then sterilized) are prepared in a clean area.
Products that are not terminally sterilized are aseptically prepared from sterile materials or sterilized by filtration before being finally packed in sterile containers. Such aseptically prepared products are required to be formulated or prepared in an aseptic area. An aseptic area is a room within a clean area designed, constructed, serviced, and used to prevent microbial contamination of the product. This is a very interesting topic from microbiology.
SOURCES OF CONTAMINATION IN ASEPTIC AREA
Atmosphere
The atmosphere is unable to support microbial growth but dust particles in outside air (originating from the soil) may carry soil microorganisms. E.g. Bacillus spp., Clostridium spp., yeasts, and molds. Indoor air may also contain staphylococcus spp., and Streptococcus spp., on particles of skin or clothing.
These microbes also occur in droplets expelled into the atmosphere from the mouth and respiratory tract by talking, sneezing, and coughing. Microorganisms free from dust particles, mainly mold spores, are commonly found in indoor and outdoor air. A damp atmosphere usually contains fewer microorganisms than a dry one as the contaminants are carried down by the droplets of moisture. Thus, the air in a cold store is usually free from microorganisms.
Operator
The skin, hair, and clothing of the operator are potent sources of microbial contamination. Microorganisms mainly present on the skin include Staphylococcus spp., lipophilic yeasts, and dermatophytes fungi. Poor personal hygiene can result in the presence of skin coliforms and other intestinal bacteria. Open wounds are a source of saprophytic and pathogenic microorganisms. The nasal passages may contain Staphylococcus aureus, Staphylococcus albus, Neissria Pharyngis etc.
Raw materials
Raw materials may account for a high proportion of microbial contamination in pharmaceutical products. Drugs that are prepared from animals, plants, or other natural sources are frequently contaminated with bacteria, yeasts, and molds. Water is also a prime source of microbial and particulate contamination.
Equipment
Equipment used for the formulation of pharmaceuticals is also a source of microbial and particulate contamination. Working surfaces and external surfaces of equipment are also potential sources of contamination due to the sedimentation of particles and droplets from the atmosphere.
BUILIDING DESIGN, CONSTRUCTION AND PRODUCTION FACILITIES
Production of sterile products should be carried out in a clean environment with a limit on the environmental quality of microbial and reduce the risk of product contamination.
The production area is normally divided into the
- the clean-up area
- the compounding area,
- the aseptic area
- the quarantine area
- the packing area
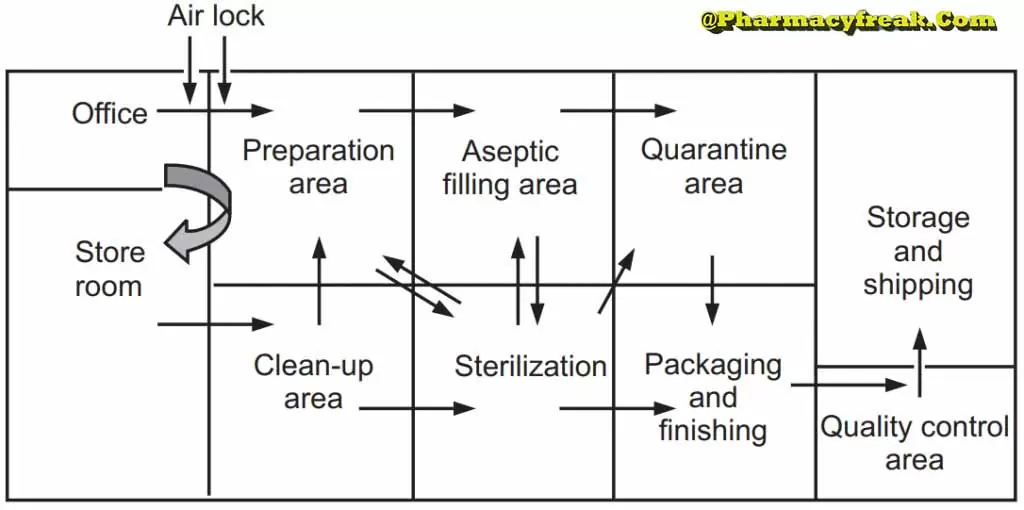
All these areas should be designed and constructed for case of cleaning, efficient operation, attractiveness, and of personnel.
Clean areas for the production of sterile products are classified into grades A, B, C, and D. These grades are categorized by the particulate quality of the environmental air when the clean area is operating in both a ‘manned’ and ‘unmanned’ state. These areas are graded by microbial monitoring of the environmental air, surface, and operators when the area is functioning.
The operation of clean and aseptic rooms is recognized by three main areas as black or dirty areas, grey or semi-clean areas, and white or clean areas. Black areas are those where particle and microbial control is almost impossible. Grey areas are those where high standards of hygiene and cleanliness operate and reduction of microbial contamination is possible. In white areas where full environmental control is operational and special protective clothing is worn by the operators. The area may be a Class-1 (aseptic) room or a Class-II(clean) room possibly with laminar flow units installed at terminal operation points such as filling lines.
| GRADE | VIABLE MICROORGANISM/M³ OF AIR | SETTLE PLATE (9CM)/4 Hrs. | CONTACT PLATE (5.5 cm) | GLOVE PRINT ( 5 FINGERS) |
| A | <1 | <1 | <1 | <1 |
| B | 10 | 5 | 5 | 5 |
| C | 100 | 50 | 25 | N/A |
| D | 200 | 100 | 50 | N/A |
The sterile production unit must be separated from the general manufacturing area within the hospital pharmacy or factory. This sterile production unit must not be accessible to unauthorized personnel. The clean room should be remote from main corridors, stairways, or lifts which provide airways for bacterial and particulate transmission. It is important to design the clean room to be the smallest size, bearing in mind the operations to be undertaken and the number of people likely to be employed in the area.
Floors walls and ceilings: All clean room surfaces including the floors, walls, and ceilings must be smooth, impervious, easily cleaned and disinfected, and be constructed to minimize microbial and particulate contamination. Flexing and non-flexing types of material are used for the construction of floors. Flexing floor materials are made of synthetic elastomers of which the most commonly used are polyvinylchloride (non-slip grades). Polyvinylchloride flooring is easily repaired, cleaned, relatively cheap, and simple. Non-flexing floors are made of hard inorganic filler substances in a matrix material. When concrete is used it must be adequately sealed with a material resistant to chemicals, solvents, and cleaning fluids.
Walls must be made of non-inflammable or fire-resistant material e.g. stainless steel, glass, enameled steel, etc. Generally, plaster walls are easily damaged by impact. For reduction of fungal growth, 1% of 8-hydroxyquinoline, pentachlorophenol, or salicyanilide may be added to the paint. Epoxy resin paints and polyurethane paints are also used to avoid cracking and peeling. The ceiling is sealed to prevent the entry of microbial kept to a minimum.
Doors, windows, and services: Doors and windows should fit flush with the walls. Windows, if required are solely to provide illumination and are not for ventilation. They should be nonopenable. Doors should be well fitted by maintaining positive pressure airflow and self-closing. There should be a limited number of entry doors for personnel and ports for materials. Airlock doors, wall ports, autoclaves, and dry heat sterilizers should be fitted with interlocked doors.
All pipes passing through the walls of the room should be effectively sealed and should be flush fitting and easily cleaned. Gas cylinders should be excluded and all gases should be piped from outside the area. Sinks and drains must be excluded from areas where aseptic procedures are performed in clean room areas. The light source in clean rooms is fitted with a ceiling to reduce the collection of dust and to avoid disturbance to the airflow pattern within the room. Nonessential switches such as room lighting switches and isolators should be installed outside the clean areas.
Personnel and protective clothing: The main source of contamination of clean areas from skin scales that are released by the operators. Personnel selected to work on the preparation of a parenteral product must be neat and reliable. They should be in good health and free from a dermatological conditions that might increase the microbial load. Operator-borne contamination can be controlled by limiting the number of operators in the clean area, restricting their movement, and isolating them from the product and the environment with suitable protective clothing. All personnel should be trained in good manufacturing practices and aseptic techniques.
Productive clothing is designed to prevent contamination from the body and everyday clothing. They should be restricted to use in aseptic or clean areas only. All protective clothing must be sterilized by moist heat sterilization or ethylene oxide sterilization. The operator must wear sterile protective clothing including headwear, powder-free rubber or plastic gloves, a non-fiber shedding facemask, and footwear. Fresh sterile clothing should normally be provided each time a person enters an aseptic area.
Cleaning and disinfection
Cleaning and disinfection procedures are used for the removal of microbial and particulate contamination. Cleaning agents are detergents and non-ionic and tonic surfactants. Different types of disinfectants should be employed in rotation to prevent the development of resistant strains of microorganisms. Different concentrations of quaternary ammonium compounds, sodium hypochloride, ethanol, and formaldehyde solution are used as disinfectants in clean areas. Cetrimide or chlorhexidine in 70% alcohol is suitable for use as skin disinfectants.
ENVIRONMENTAL CONTROL
Potential sources of microbial and particle contaminants occurring within the clean room are the air supply of the room, the inflow of external air, and production contaminants within the room. It can be seen that the British Standard Class 2 for environmental cleanliness closely approximates to US Federal Standard Class 10,000 particles per cubic foot of size 0.5 pm or greater, shall be found in the measured areas. The more stringent British Standard Class 1 approximates US Federal Class 100 and is the standard Class 100 and is the standard applicable to aseptic areas.
Air Supply
The air supplied to a clean room must be filtered through high-efficiency particulate air (HEPA) filters. The HEPA filter must be positioned at the inlet for the clean room and the pre-filter may be fitted upstream of the HEPA filter to prolong the life of the final filter. In these HEPA filters, pleated fiberglass paper is used as the filter medium. The final filter consists of a continuous sheet of filtration material, pleated with a corrugated separator placed between the HEPA filter.
Laminar air flow equipment can deliver clean air in a vertical, horizontal, or curvilinear direction. The air filtered from laminar airflow is claimed to be 99.97% free of microbial contamination. This level is based upon the removal of dioctyl phthalate (DOP) particles of size 0.3 um and larger. Air velocity at all parts of the filter should be 90 ±20 feet/min (0.54m/sec.)
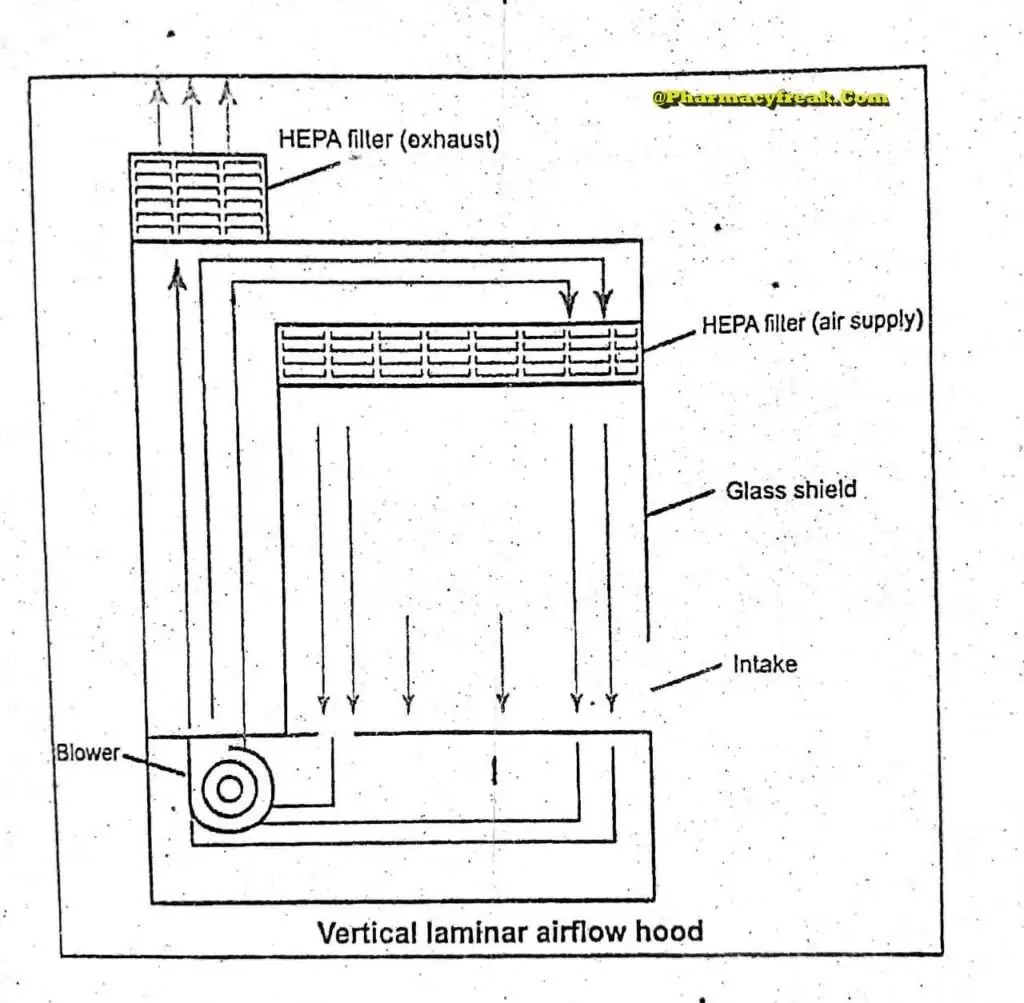
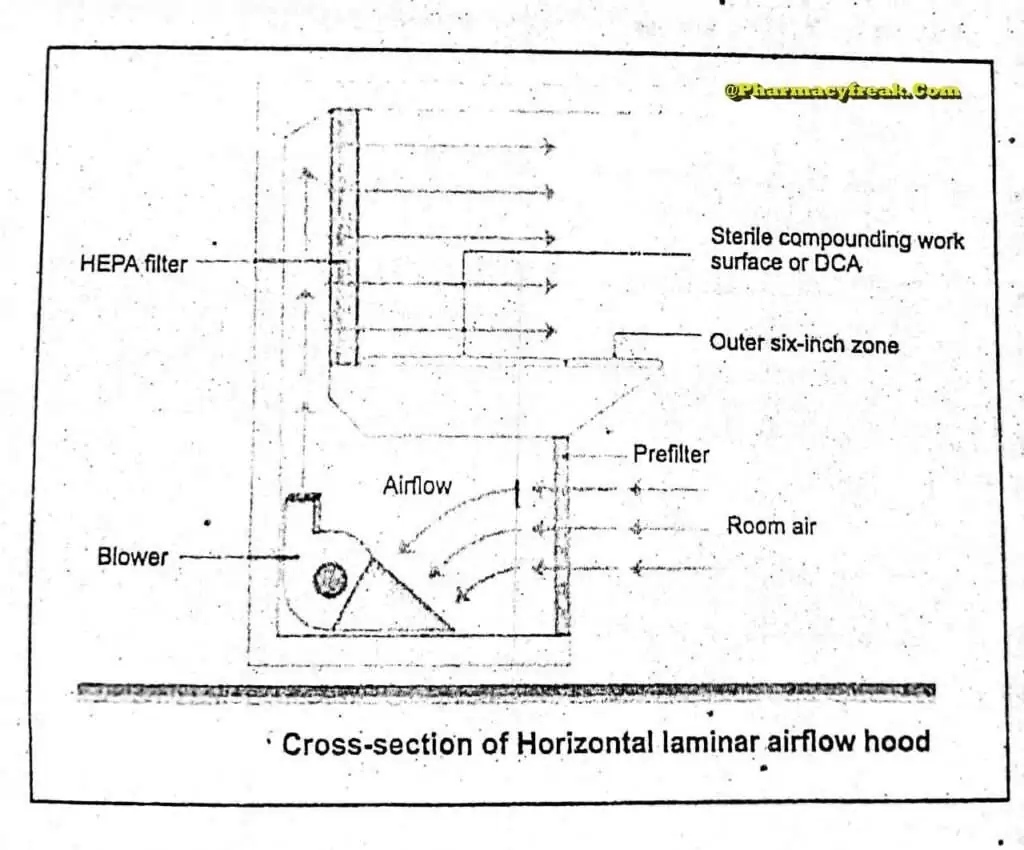
Quality control procedure must be adopted to evaluate and monitor the quality of the HEPA filter. These filter are supported to provide class 100 air and they should be certified after every 6 to 12. months. Air quality is evaluated using settle plates, microbial air samplers and particle counters. The velocity of HEPA- filtered air is measured using an air velometer. The efficiency of the filter in removing particulate and microbial contamination is evaluated by employing the dioctylphthalte (DOP) test.
This is a universally acceptable challenge test for HEPA filters. Dioctyphthalate smoke is introduced at the supply plenum and photometer probe scan the surface of the filter for smoke particles. Dioctylphthalle is a volatile liquid and under pressure, it converts to vapor or smoke having a size range of 0.4 um Leakage of the filter will permit the dioctylphthalte particles to escape and this will be detected by the photometer. Sensors are fitted upstream and downstream the filters to indicate the pressure differential across the filter. An alarm system should be used to indicate failure in the air supply or filter blockage.
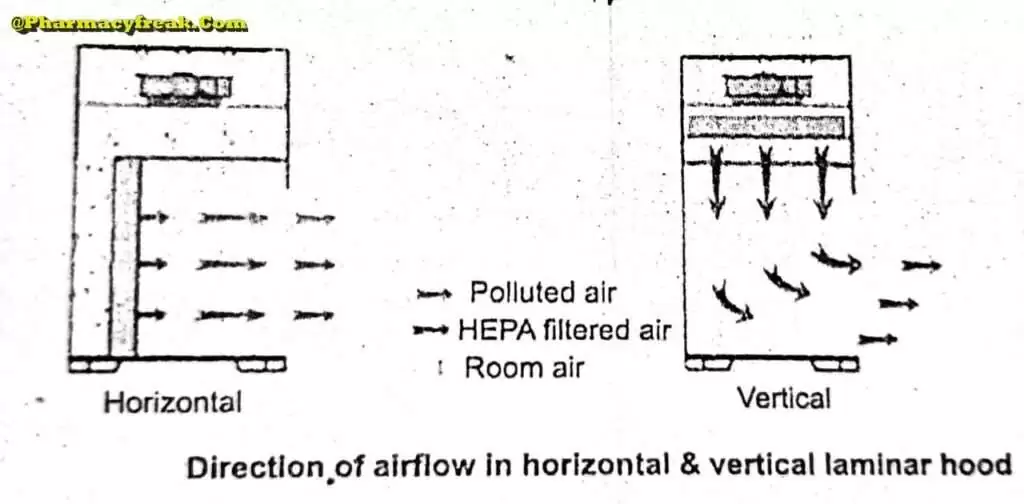
Airflow Pattern
The airflow pattern within the clean room must be carefully regulated to avoid generating particles from the clean room floors, walls, and operators. The general airflow patterns in clean rooms are unidirectional airflow, non-unidirectional airflow, and combination airflow.
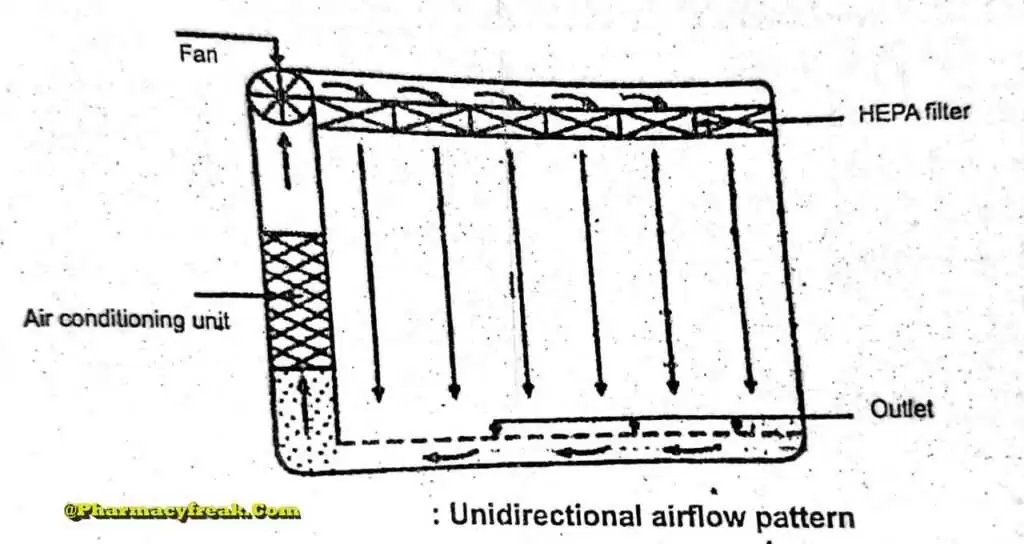
Unidirectional Airflow
The air within the room moves with uniform velocity along parallel flow lines. Air enters the room through a bank of filters and exits through a bank of outlets comprising the opposite wall or floor. The velocity of the air is highly 0.3 m/s in downflow air from ceiling filters and 0.45 m/s in cross-flow air. These are highly efficient airflow systems but expensive to construct. Because of operating costs, unidirectional airflow clean rooms are not often used in pharmaceuticals.
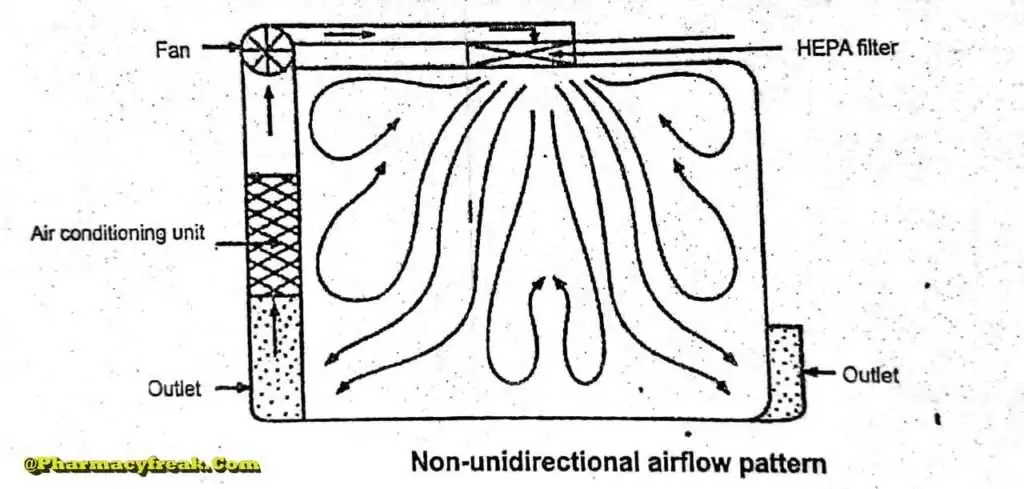
Non- unidirectional airflow
The air enters the clean rooms through filters and exits through outlet ducts, positioned low down on the wall or the floor at sites remote from the air inlet. The filtered inlet air mixes with and dilutes the contaminated air within the room. Conventional airflow is defined in terms of the number of air changes per hour ( <_20 air charges/hour).
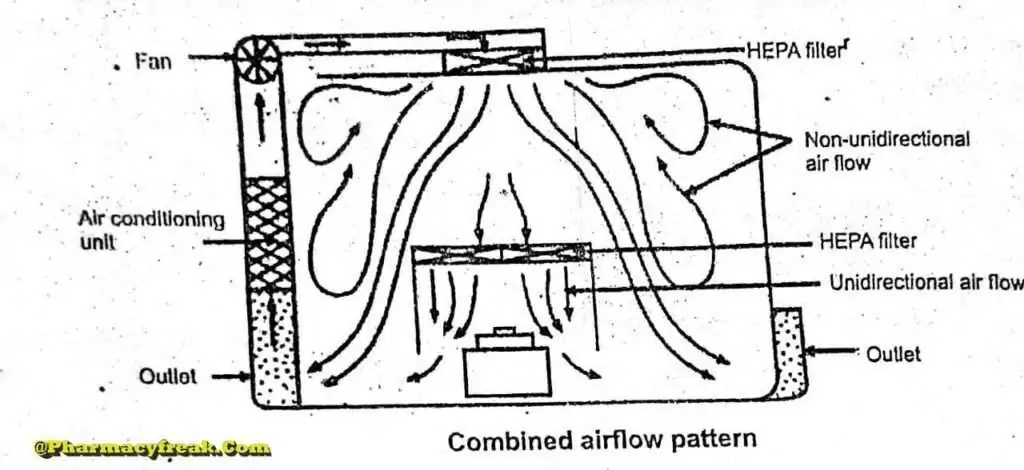
Combined Airflow
In many pharmaceutical clean rooms, the background area is ventilated by a non- unidirectional airflow and critical areas are supplied with unidirectional airflow . Various vertical unidirectional airflow systems are used in combination clean rooms. There has been a trend towards protecting the critical procedures within combination clean rooms by using isolator cabinets.
The temperature and the humidity are adjusted to suit the procedures being carried out within the clean room. A temperature of about 20 to 22°C with a relative humidity of about 35 to 50% are often preferred.
TESTING OF CLEAN AND ASEPTIC ROOMS
To provide satisfactory conditions for proper processing of parenteral, it is necessary to monitor the area with suitable environment control tests. These methods can be divided tests general methods, sampling methods, and surface sampling methods.
General Methods
General methods are used to validate HEPA filters, detect particulate contamination, and monitor the environment. These methods include filter efficiency tests,
induction leak test, particulate contamination control test, air pressure test, air flow tests, noise
level tests, lighting tests, and temperature and humidity tests.
Air Sampling methods
Air sampling methods include
- Electronic air particle counters
- Settle plates
- Slit-air sample
- Liquid Impinger
- Centrifugal air sample (Biotest)
Electronic air particle counters
These counters are especially useful in determining the number of particles/microbes counted per cube feet to classify the cleanliness of a particular room or area. Electronic counter all particles/microbes but cannot differentiate between viable and non-viable microbes.
Settle plates
It is also called the sedimentation method & is based on the principle of measuring the rate at which bacteria-carrying particles settle down. Open plates of nutrient agar or other suitable medium are exposed to the atmosphere in the sampling area for a predetermined period of 30 to 60 minutes. Plates are incubated at 30-37°C for 48 hours and colonies are counted. This method is simple and inexpensive & is commonly used for testing the air in surgical theaters and hospital wards. The major disadvantage of this method is that only those microorganisms that adhere to the surface of large particles are counted.
Slit-air sample
This method is better than the settle plate method. The efficiency of detection even small particles containing organisms is quite high. The slit air sample is one of the most widely used monitoring methods for manufacturing parenteral and quality control environments. It gives a count of bacteria carrying particles contained in a given volume of air. In this method a known volume of air is directed onto a plate of culture medium through a slit of 0.25 mm width, the plate being mechanically rotated to ensure uniform distribution of organisms all over the plate containing medium. One cubic foot of air is allowed to pass through the slit and likewise, 10 cubic feet of air is tested. The culture media are incubated and colonies counted which gives the bacterial number present in the air.
Liquid Impinger
The air sample may be drawn into a measured volume of nutrient broth in an impinger. Microorganisms in the broth, then may be collected by membrane filtration, incubated and counted.
Centrifugal air sample (Biotest)
Airborne microorganisms approximately 16 inches above the sterile drum housing are drawn toward the impeller blades. By applying centrifugal forces (approx. 4000 to 4200 rpm) the microbial particles are impacted at a high velocity onto the incubation, colonies on the strips are counted and recorded (CFU/unit volume of air).
Surface sampling methods
- Rodac plates- Samples of the level of microorganisms on the surface can be determined by specially built convex surface Petri plates, With the Rodac plates it in possible to roll the agar surface over a flat or irregular surface to be tested. Surface contamination can be quantified by counting the colonies after incubation at 30 to 35 degrees for 48 hours
- Swab- rinse test- This is a simple surface sample method employing sterile cotton swabs to sample locations. The swab is then placed into tubes of culture media or sterile water (microbial counting) and a sample of the water is placed on a solid plate
The purpose of environment control of microbial contamination is to minimize the potential for product contamination. The lesser the potential for contamination, the greater the arrange that the product is sterile. The sterility test can be used primarily as a confirmation of the sterility already built into the product.
Bacteriological Examination of Environment Dust
Air Centrifugation
- This involves the principle of centrifuging particles on a culture medium borne on a plastic strips.
- After sampling the strip is removed from the instrument and incubated at 37″ C for 48 hours and colonies are counted.
- However, this method is less efficient for particles less than 5um in size.
- This method is inferior to the slit sampler method.
Sweep Plate Method
- Useful for detection of organisms from personal clothing, bedclothes, and curtains having bacteria-laden dust.
- An open culture plate is swept 8 to 10 times over the dusty surface with a swab soaked in nutrients and culture plate incubated and clonies counted after overnight incubation.
Dust Sampling
- Moistened cotton wool swabs can be used for collecting dust from the floor, wall, furniture, etc.
- The swabs are placed in broth as well as in Robertson-cooked meat media and Incubated.
- After sub-culturing on plates, the organisms can be identified.
- This is used as a routine for reviewing the level of asepsis in the surgical theater, especially for demonstration of spores of Clostridium tetani and other spore bearers in theater dust (in positive cases fumigation of theater is advised and theaters closed till bacteriological report shows negative results regarding Clostridium tetani).
Interpretation
- Most bacteria found in the air are harmless saprophytes or commensals.
- About 1 percent of airborne organisms in the wards or closed rooms are harmful.
- Streptococcus may be found in large numbers, ie, 10 per cubic foot in a room occupied by streptococcal infected patients like streptococcal tonsilitis or scarlet fever.
- Staphylococcal aureus is the predominant airborne organism (0.1 to 10 organisms per 1 cube foot of air).
- A man may be infected even when he inhales only one Mycobacterium tuberculosis in 500 cubic feet of air that he respires for 24 hours.
- Air contamination standards are as follows.
- Operation theater, etc. should have not more than 10 per cubic foot.
- Dressing rooms, operation theaters for neurosurgery, and theaters catering burn patients the bacterial count should not exceed 1 per cubic foot.
- Factories, offices, and homes should have a count below 50 per cubic foot.

I am a Registered Pharmacist under the Pharmacy Act, 1948, and the founder of PharmacyFreak.com. I hold a Bachelor of Pharmacy degree from Rungta College of Pharmaceutical Science and Research. With a strong academic foundation and practical knowledge, I am committed to providing accurate, easy-to-understand content to support pharmacy students and professionals. My aim is to make complex pharmaceutical concepts accessible and useful for real-world application.
Mail- Sachin@pharmacyfreak.com