Table of Contents
Introduction
Atomoxetine is a selective norepinephrine reuptake inhibitor (NRI) approved for the treatment of Attention-Deficit/Hyperactivity Disorder (ADHD). It is a non-stimulant alternative to conventional stimulants like methylphenidate and amphetamines, especially beneficial for patients at risk of substance abuse or with contraindications to stimulants.
Mechanism of Action (Stepwise)
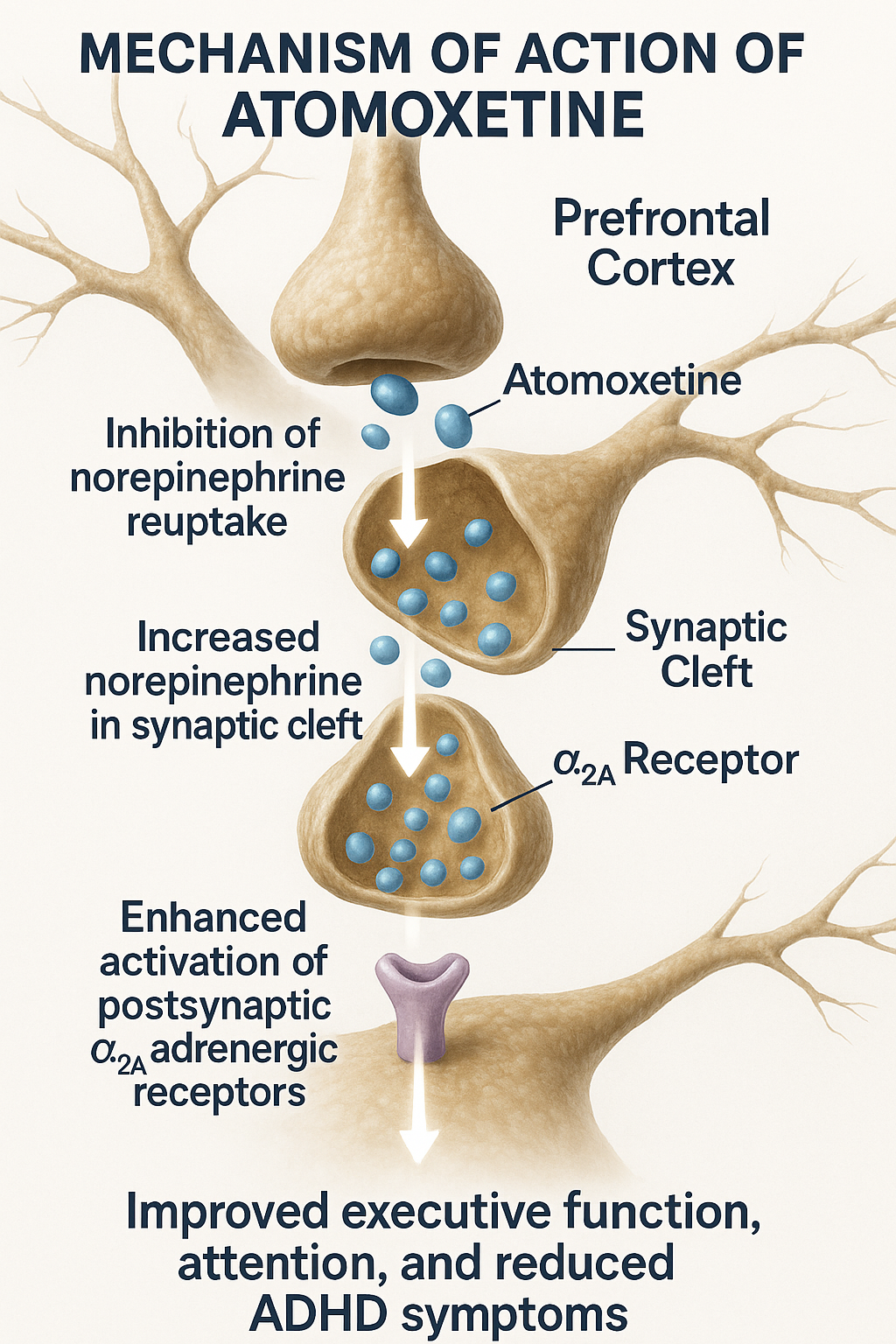
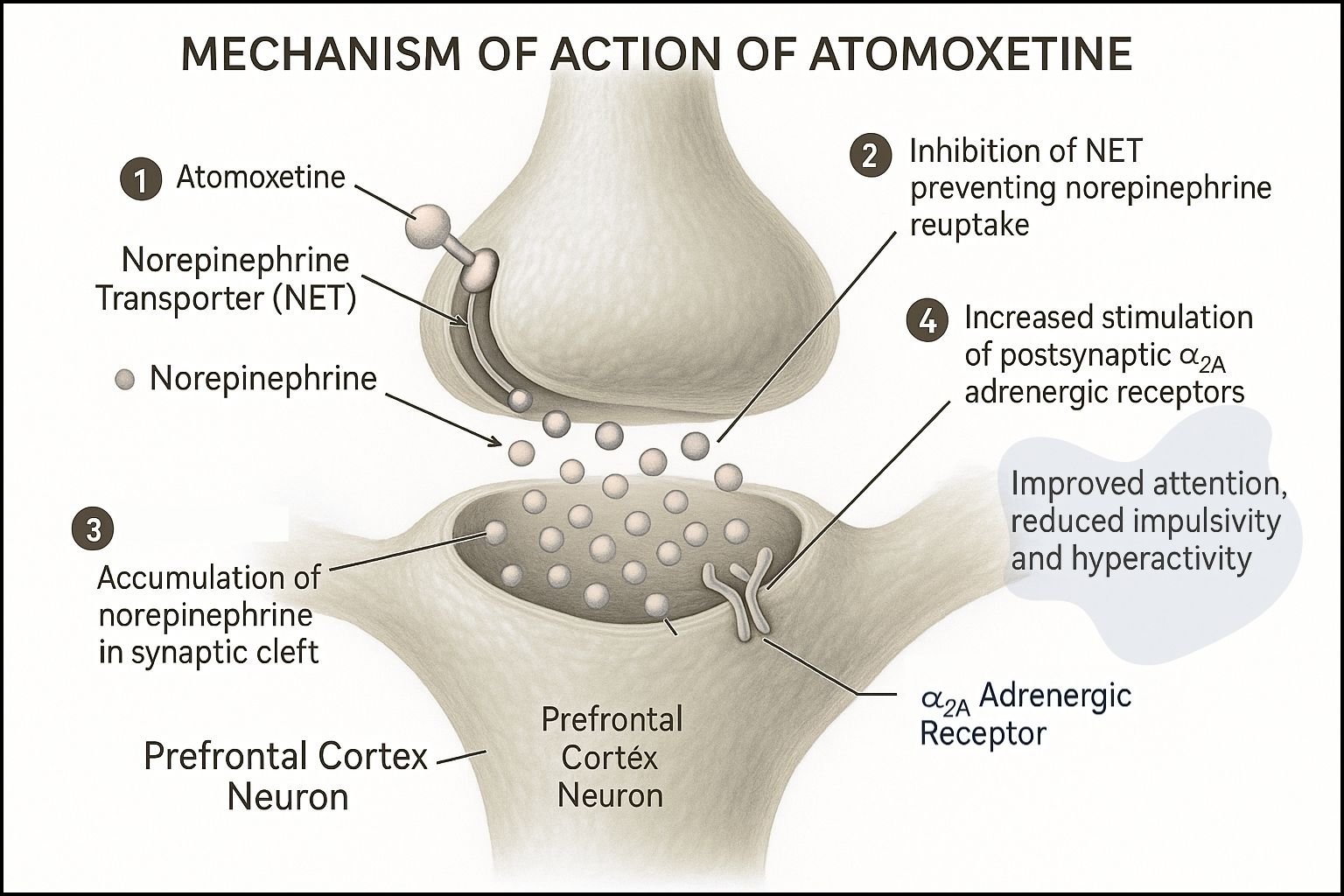
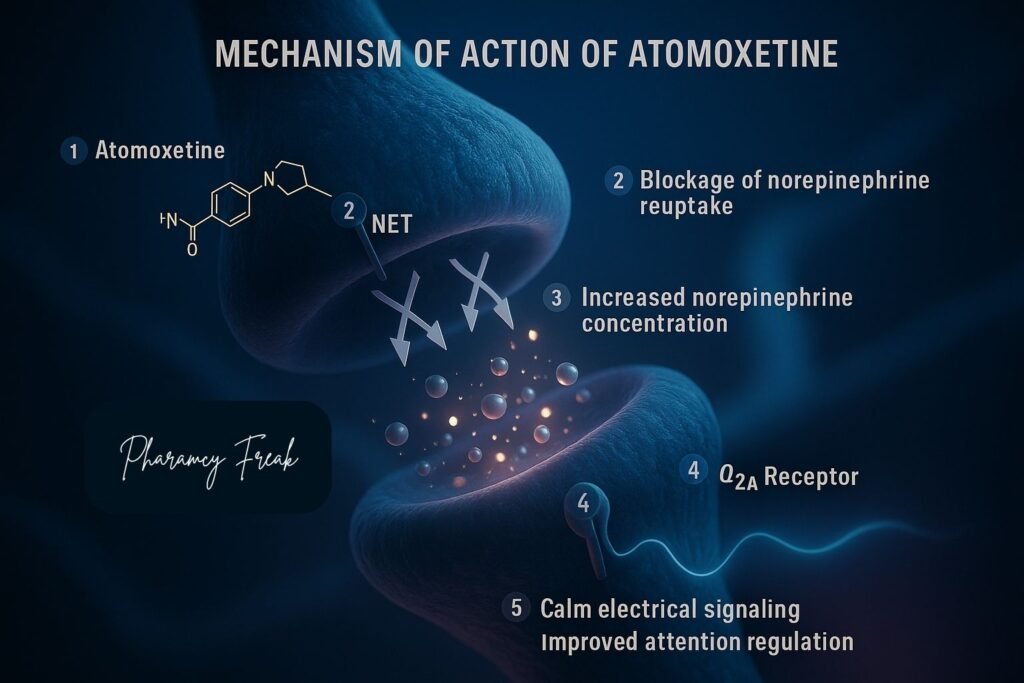
- Selective Inhibition of Norepinephrine Transporter (NET)
- Atomoxetine selectively inhibits the presynaptic norepinephrine transporter.
- This action blocks the reuptake of norepinephrine from the synaptic cleft back into the presynaptic neuron.
- Increased Extracellular Norepinephrine Levels
- By blocking reuptake, atomoxetine increases norepinephrine concentration in the prefrontal cortex and other brain regions associated with attention and impulse control.
- Minimal Effect on Dopamine Transporter (DAT)
- Atomoxetine has negligible affinity for DAT in the striatum, hence reduced abuse potential and minimal euphoric effects.
- Delayed Onset of Therapeutic Effects
- Unlike stimulants, the full clinical benefit of atomoxetine may take 1–4 weeks to manifest.
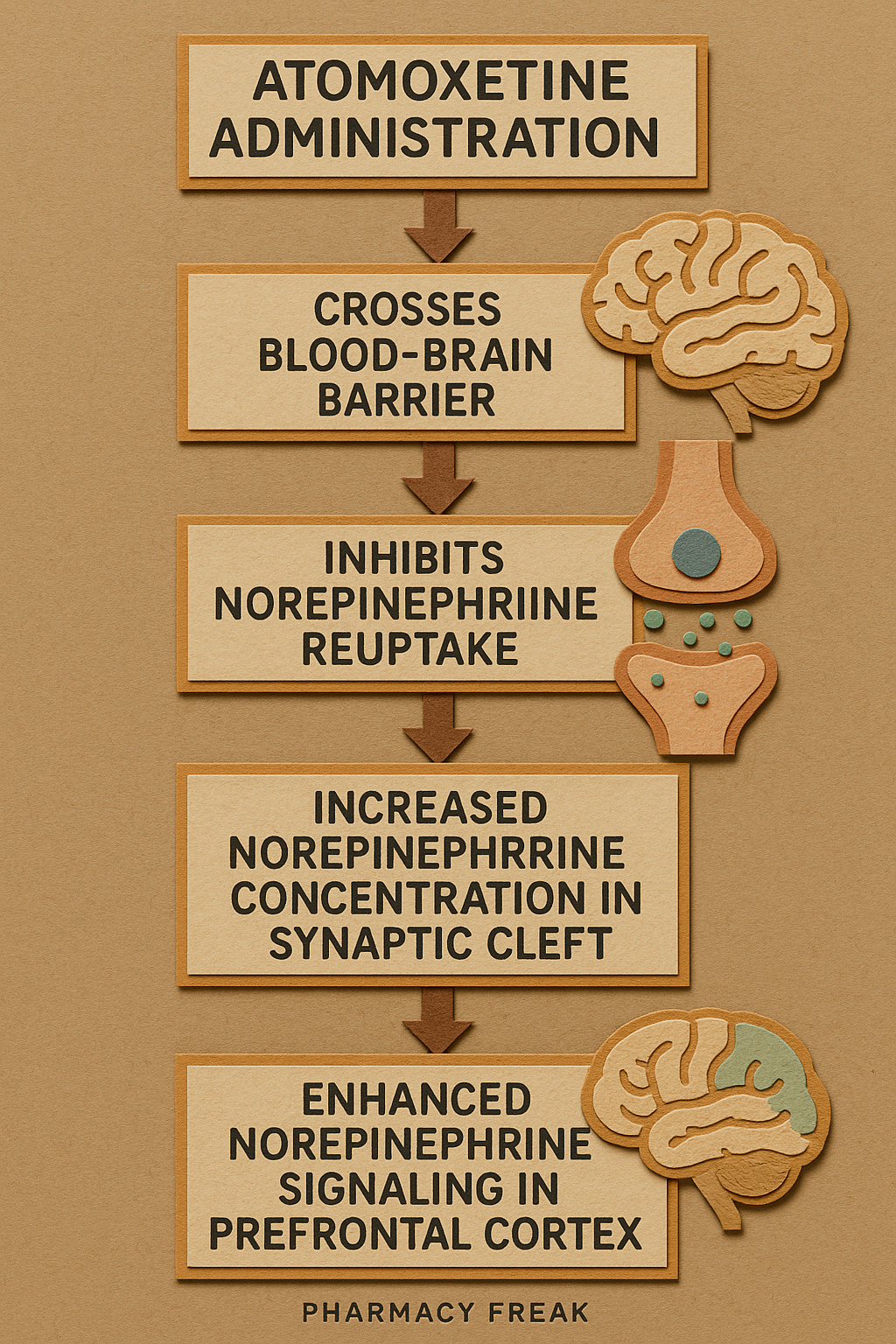
Pharmacokinetics
- Absorption: Rapidly absorbed; peak plasma concentrations within 1–2 hours.
- Bioavailability: 63% (extensive first-pass metabolism).
- Metabolism: Primarily via CYP2D6; genetic polymorphism affects plasma levels.
- Half-life:
- Extensive metabolizers: ~5 hours
- Poor metabolizers: ~24 hours
- Excretion: Mainly via urine (80%), as metabolites.
Clinical Uses
- Primary indication: ADHD in children, adolescents, and adults.
- Off-label: May be used in depression with executive dysfunction or comorbid ADHD.
Adverse Effects
- Common: Dry mouth, insomnia, nausea, decreased appetite, dizziness.
- Serious: Suicidal ideation (especially in children), hepatotoxicity, severe allergic reactions.
- Monitoring: Liver function tests (rare but severe liver injury), cardiovascular status in at-risk patients.
Comparative Analysis
| Feature | Atomoxetine | Methylphenidate |
|---|---|---|
| Drug Class | NRI | CNS stimulant |
| Abuse Potential | Low | Moderate to high |
| Onset of Action | Delayed (1–4 weeks) | Rapid (within hours) |
| Mechanism | NET inhibition | DAT and NET inhibition |
| First-line for ADHD | Yes | Yes |
| Cardiovascular Risk | Moderate | Moderate to high |
Atomoxetine is suitable for patients where stimulant use is contraindicated, or where substance abuse risk is a concern.
Multiple Choice Questions
- Atomoxetine primarily inhibits:
- A. Dopamine reuptake
- B. Serotonin reuptake
- C. Norepinephrine reuptake
- D. Monoamine oxidase
- Answer: C. Norepinephrine reuptake
- Atomoxetine differs from stimulants in that it:
- A. Enhances dopamine levels
- B. Has immediate effect
- C. Inhibits norepinephrine reuptake
- D. Is a dopamine agonist
- Answer: C. Inhibits norepinephrine reuptake
- The major metabolic enzyme for atomoxetine is:
- A. CYP3A4
- B. CYP2D6
- C. CYP1A2
- D. CYP2C19
- Answer: B. CYP2D6
- Which of the following is an advantage of atomoxetine over stimulants?
- A. Faster onset
- B. No abuse potential
- C. Higher efficacy
- D. No need for metabolism
- Answer: B. No abuse potential
- Atomoxetine is indicated for:
- A. Schizophrenia
- B. Bipolar disorder
- C. ADHD
- D. Parkinson’s disease
- Answer: C. ADHD
FAQs
Q1. Is atomoxetine a stimulant?
No, it is a non-stimulant NRI used for ADHD management.
Q2. How long does atomoxetine take to work?
Onset typically ranges from 1 to 4 weeks.
Q3. Can atomoxetine be used in adults?
Yes, it’s approved for use in both pediatric and adult patients with ADHD.
Q4. What makes atomoxetine safer in substance use disorders?
It lacks dopaminergic stimulation in reward pathways, reducing abuse risk.
Q5. Does atomoxetine require titration?
Yes, starting with a low dose and gradual titration is standard to minimize side effects.
References
- Goodman & Gilman’s The Pharmacological Basis of Therapeutics, 12th Edition
- KD Tripathi Essentials of Medical Pharmacology, 7th Edition
- FDA Atomoxetine Prescribing Information
- Stahl’s Essential Psychopharmacology

I am pursuing MBA in pharmaceutical management from NIPER Hyderabad with a strong academic record and proven success in national-level pharmacy entrance exams. I secured AIR 61 in NIPER 2024 (MS/M.Pharm) and AIR 27 in NIPER MBA, along with AIR 147 in GPAT 2024 and AIR 907 in GPAT 2023. I also achieved AIR 6 in AIIMS CRE-2025 for Drug Store Keeper and was selected as a Pharmacist (AIR 61) for ESIC. Additionally, I was the Runner-Up in Round 2 of the EY Case Study Competition.
At PharmacyFreak.com, I aim to guide future pharmacists through expert content, exam strategies, and insightful resources based on real experience and academic excellence.
Mail- harsh@pharmacyfreak.com