Table of Contents
Introduction
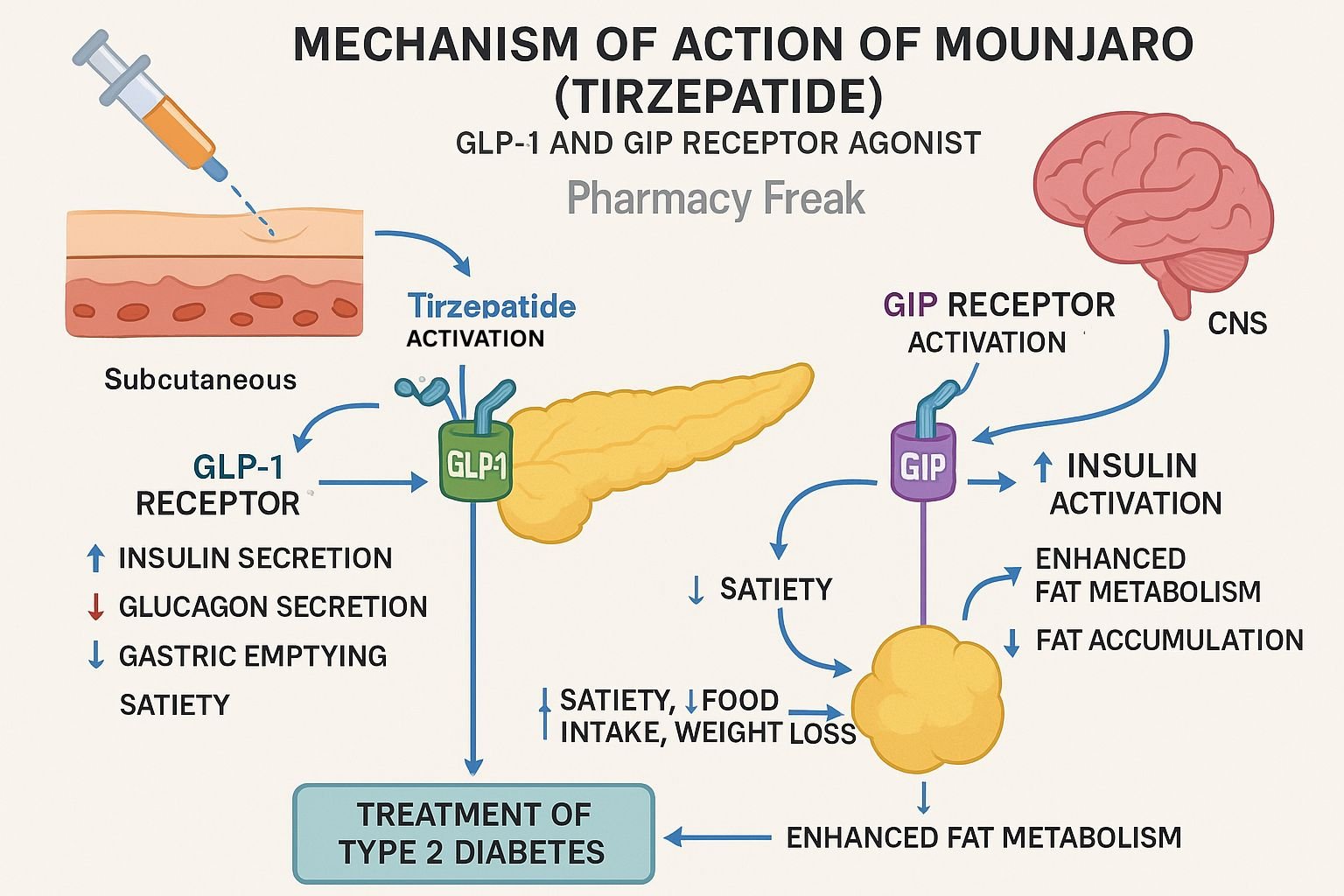
Tirzepatide, marketed as Mounjaro for type 2 diabetes and Zepbound for obesity, is a dual GIP/GLP‑1 receptor agonist. This innovative “twincretin” enhances insulin secretion, suppresses glucagon, delays gastric emptying, and promotes satiety. It offers significant HbA₁c reduction and weight loss advantages over GLP‑1 agonists alone.
Step-by-Step Mechanism of Action
- Co‑agonism at GIP and GLP‑1 receptors
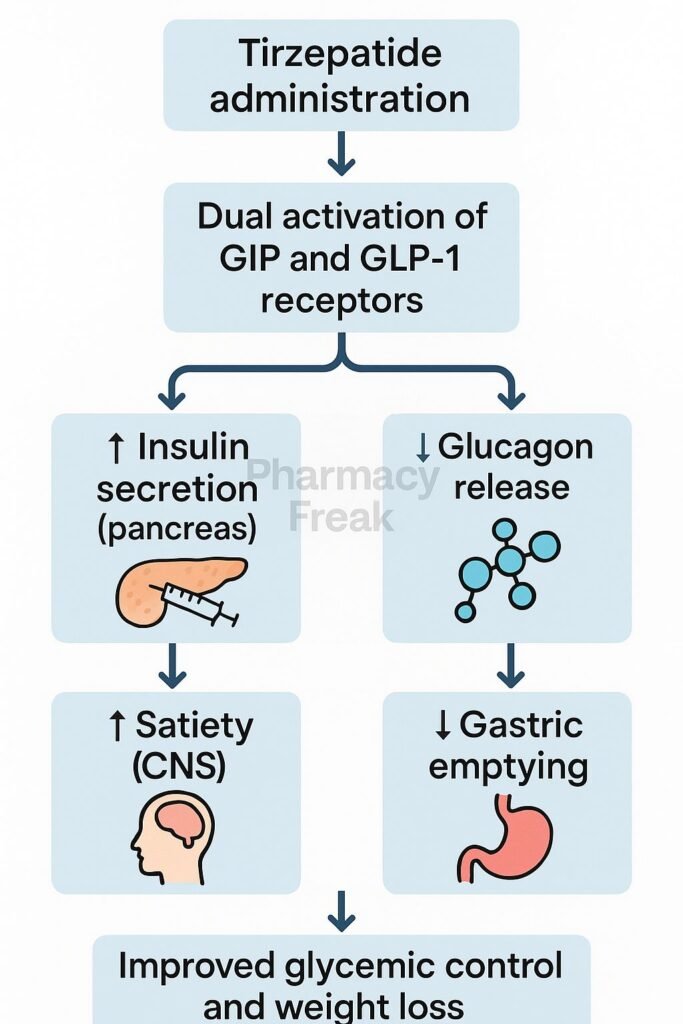
Tirzepatide binds both the GIP receptor and GLP‑1 receptor, stimulating insulin release in a glucose-dependent manner. - Enhanced insulin secretion & glucagon suppression
Activation of these receptors increases cAMP, boosting insulin exocytosis and inhibiting glucagon secretion, thereby lowering blood glucose. - Delayed gastric emptying & appetite reduction
Activation of GLP‑1 receptors in the gut slows gastric emptying and suppresses hunger, increasing satiety. - Improved β‑cell function and insulin sensitivity
Dual agonism promotes β‑cell proliferation and survival, increases adiponectin, and enhances peripheral insulin sensitivity. - Weight loss via central and peripheral effects
Beyond appetite control, GIP receptor activation amplifies weight reduction and metabolic improvements.
Pharmacokinetic Parameters
| Parameter | Value |
|---|---|
| Route | Subcutaneous injection, once weekly |
| Bioavailability | ~80% |
| Time to Peak | ~1–2 days |
| Half-life | ~5 days |
| Metabolism | Peptidase cleavage and beta‑oxidation |
| Excretion | Renal and fecal |
Clinical Uses
- Type 2 diabetes mellitus: significant HbA₁c reduction
- Obesity management: substantial weight loss
- Prevention of prediabetes progression
Adverse Effects
- Gastrointestinal: nausea, vomiting, diarrhea, constipation
- Neurological: headache, decreased appetite
- Rare: thyroid C‑cell tumors (contraindicated in MEN2), hypoglycemia with insulin or sulfonylureas
Comparative Analysis
| Agent | Receptor Target | HbA₁c Reduction | Weight Loss |
|---|---|---|---|
| Tirzepatide | GIP + GLP‑1 agonist | 1.2–2.6% | 5–21% |
| Semaglutide | GLP‑1 agonist | ~1.5–2.0% | ~15% |
MCQs
- Tirzepatide acts by agonizing which receptors?
a) GIP only b) GLP‑1 only c) GIP + GLP‑1 d) Insulin
Answer: c) GIP + GLP‑1 - Its insulinotropic effect is dependent on:
a) Fasting state b) Glucose levels c) Glycogen stores d) Amino acids
Answer: b) Glucose levels - Dual action gives it better ______ compared to GLP‑1 agonists.
a) BP control b) Weight loss c) Hypoglycemia risk d) Lipid control
Answer: b) Weight loss - Common GI side effects include:
a) Rash b) Nausea c) Hypertension d) Tachycardia
Answer: b) Nausea - The half-life is approximately:
a) 12 hours b) 5 days c) 24 hours d) 2 weeks
Answer: b) 5 days - Dosing frequency is:
a) Daily b) Weekly c) Monthly d) PRN
Answer: b) Weekly - Metabolism involves:
a) CYP3A4 b) Peptidase cleavage c) Renal excretion
Answer: b) Peptidase cleavage - Dual agonism enhances ______ more than GLP‑1 alone.
a) β‑cell survival b) Muscle mass c) BP control d) Digestion
Answer: a) β‑cell survival - Weight loss seen is up to:
a) 5% b) 10% c) 15% d) 21%
Answer: d) 21% - Prediabetes progression risk reduced by ~:
a) 50% b) 70% c) 90% d) 94%
Answer: d) 94% - Most common adverse effects are:
a) Hypoglycemia b) GI issues c) Cough d) Edema
Answer: b) GI issues - Contraindicated in MEN2 due to risk of:
a) Pancreatitis b) Thyroid tumors c) Hypertension d) DVT risk
Answer: b) Thyroid tumors - Compared to semaglutide, tirzepatide is:
a) Less effective b) More effective c) Same weight loss d) Not for diabetes
Answer: b) More effective - Appetite reduction is due to:
a) GLP‑1 action b) GIP action c) Insulin action d) Glucagon suppression
Answer: a) GLP‑1 action - Mounjaro is administered:
a) Oral pill b) SC injection c) IV d) Patch
Answer: b) SC injection
FAQs
1. How quickly does Mounjaro reduce HbA₁c?
Expect a 1–2% reduction within 12–16 weeks after dose escalation.
2. What is the administration method and frequency?
Once-weekly subcutaneous injection, similar to GLP‑1 agonists.
3. Is thyroid monitoring required?
Yes—contraindicated in MEN2 or medullary thyroid cancer; monitor for neck swelling.
4. Can tirzepatide be combined with insulin?
Yes, but insulin dosage may need adjustment to prevent hypoglycemia.
5. Why use dual agonist instead of GLP‑1 alone?
Because GIP receptor activation complements GLP‑1 effects, enhancing glycemic, weight, and β-cell benefits.
References
- Goodman & Gilman’s The Pharmacological Basis of Therapeutics, 13th Edition
- KD Tripathi. Essentials of Medical Pharmacology, 8th Edition
- Cardiovascular Diabetology: Tirzepatide dual agonist review
- DrugBank: Tirzepatide pharmacology summary
- StatPearls: Tirzepatide overview
- JCI Insight: Early clinical outcomes of tirzepatide
- Frontiers in Endocrinology: Safety review of tirzepatide
- JAMA Network Open: Tirzepatide vs semaglutide outcomes

I am pursuing MBA in pharmaceutical management from NIPER Hyderabad with a strong academic record and proven success in national-level pharmacy entrance exams. I secured AIR 61 in NIPER 2024 (MS/M.Pharm) and AIR 27 in NIPER MBA, along with AIR 147 in GPAT 2024 and AIR 907 in GPAT 2023. I also achieved AIR 6 in AIIMS CRE-2025 for Drug Store Keeper and was selected as a Pharmacist (AIR 61) for ESIC. Additionally, I was the Runner-Up in Round 2 of the EY Case Study Competition.
At PharmacyFreak.com, I aim to guide future pharmacists through expert content, exam strategies, and insightful resources based on real experience and academic excellence.
Mail- harsh@pharmacyfreak.com