Table of Contents
Introduction
Capecitabine is an oral prodrug of 5-fluorouracil (5-FU) widely used in the treatment of breast cancer, colorectal cancer, and gastric cancers. Designed for tumor-selective activation, it delivers higher concentrations of active 5-FU to tumor cells while reducing systemic toxicity.
The Mechanism of Action of Capecitabine centers on its stepwise conversion into 5-FU inside tumor tissues, followed by inhibition of thymidylate synthase, incorporation into DNA and RNA, and subsequent disruption of cancer cell replication.
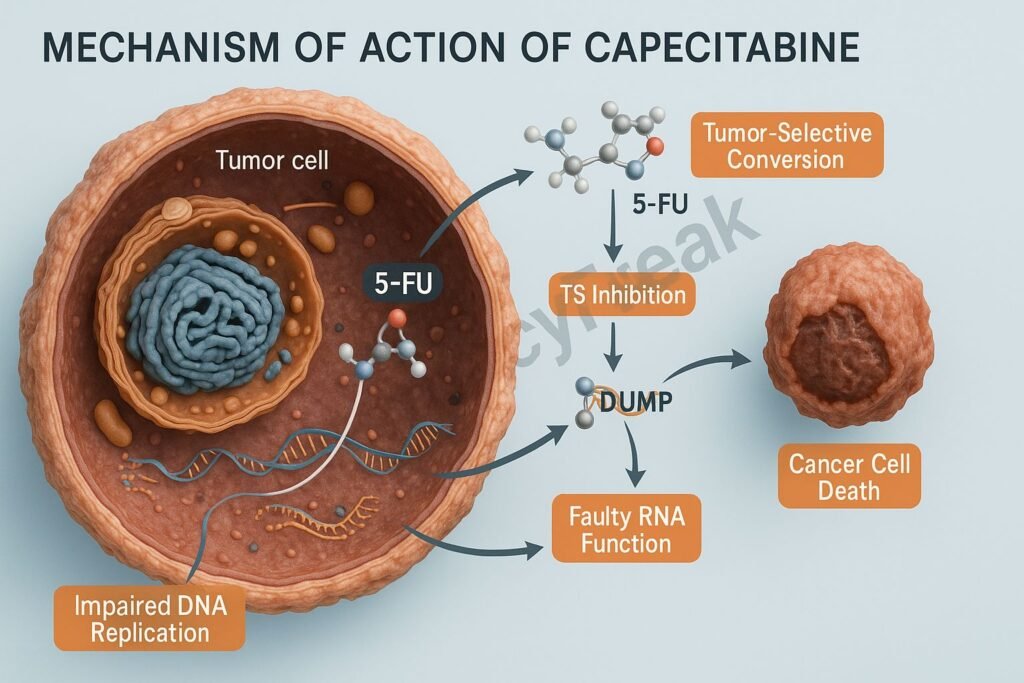
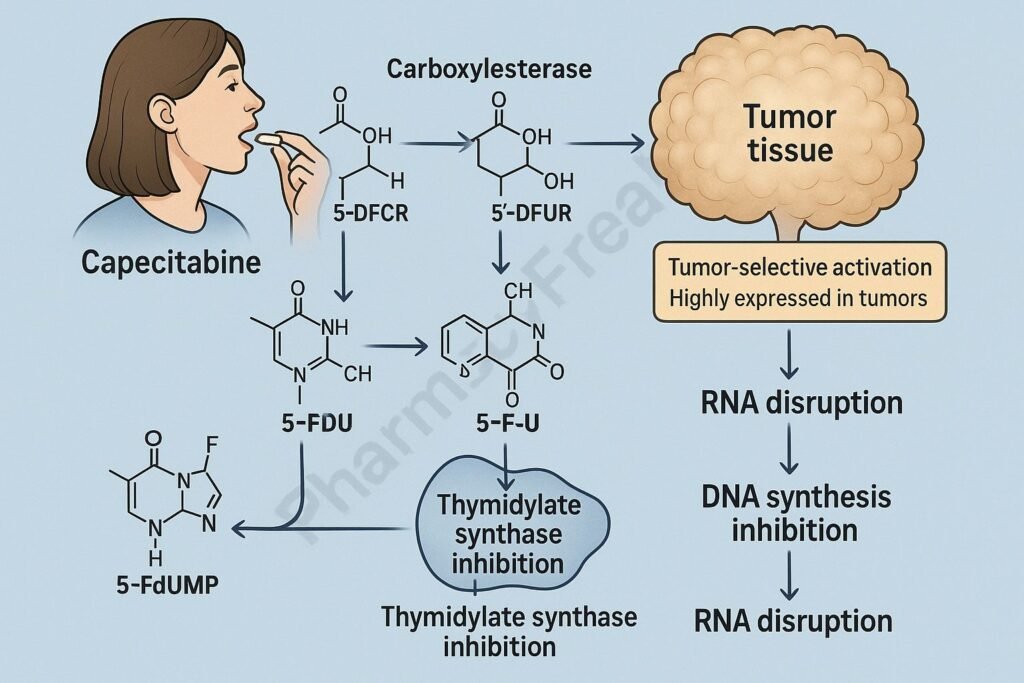
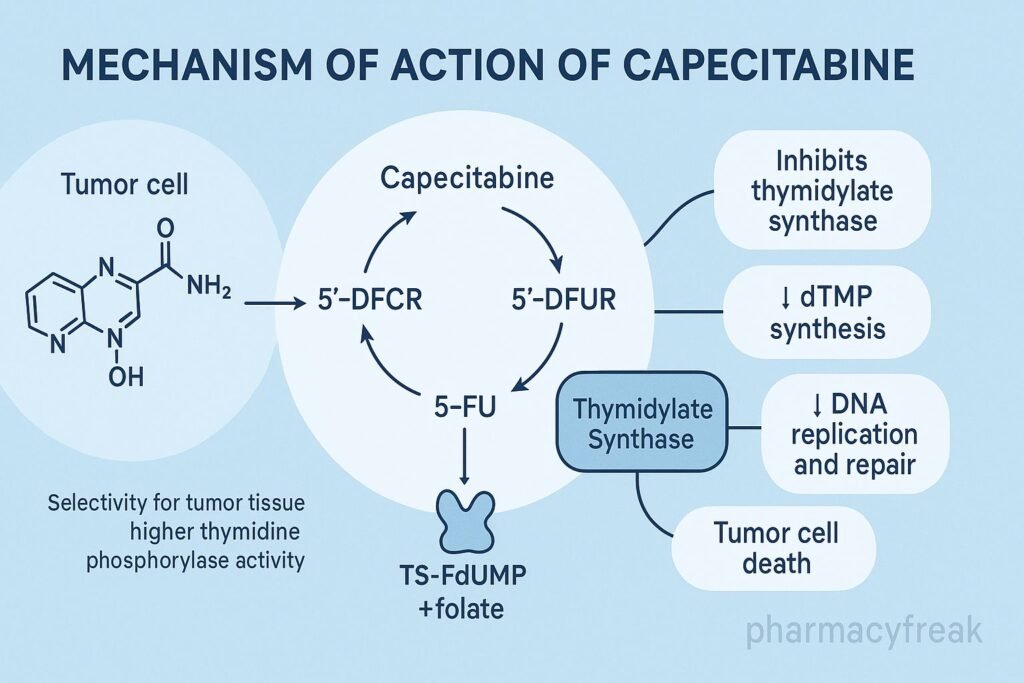
Mechanism of Action (Step-wise)
1. Oral Prodrug Conversion to 5-Fluorouracil (5-FU) – Primary Mechanism
Capecitabine undergoes a three-step enzymatic process:
- Carboxylesterase (liver)
Capecitabine → 5’-deoxy-5-fluorocytidine (5’-DFCR) - Cytidine deaminase (liver + tumor tissue)
5’-DFCR → 5’-deoxy-5-fluorouridine (5’-DFUR) - Thymidine phosphorylase (high in tumors)
5’-DFUR → 5-Fluorouracil (active drug)
Tumor-selective activation occurs because thymidine phosphorylase is highly expressed in cancer cells.
2. Inhibition of Thymidylate Synthase (TS)
Once converted to 5-FU, it forms 5-fluoro-2′-deoxyuridine monophosphate (FdUMP).
This binds to:
- Thymidylate synthase
- Reduced folate (MTHF)
Creating a stable ternary complex.
Effect:
- ↓ dTMP (deoxythymidine monophosphate) synthesis
- ↓ DNA synthesis
- ↓ Cell proliferation
- S-phase cell cycle arrest
This is the main cytotoxic mechanism.
3. Incorporation into RNA (Fraudulent RNA Synthesis)
Another metabolite, 5-fluorouridine triphosphate (FUTP), incorporates into RNA.
Results:
- Faulty RNA processing
- Impaired ribosomal function
- Disruption of protein synthesis
Particularly lethal to rapidly dividing tumor cells.
4. Incorporation into DNA
5-FU → FdUTP, which incorporates into DNA.
Effects:
- DNA strand instability
- Inhibition of DNA repair
- Fractured replication forks
- Apoptosis
5. Selective Tumor Cytotoxicity
Capecitabine is selectively activated within tumor cells due to:
- ↑ Thymidine phosphorylase expression
- ↑ Cytidine deaminase in tumors
- Lower activation in normal tissues
This improves the therapeutic index compared to IV 5-FU.
6. Summary of Mechanism
| Mechanism | Effect |
|---|---|
| Conversion to 5-FU | Tumor-selective activation |
| Thymidylate synthase inhibition | ↓ DNA synthesis |
| Incorporation into RNA | Faulty RNA → cell death |
| Incorporation into DNA | DNA damage |
| S-phase arrest | Tumor growth inhibition |
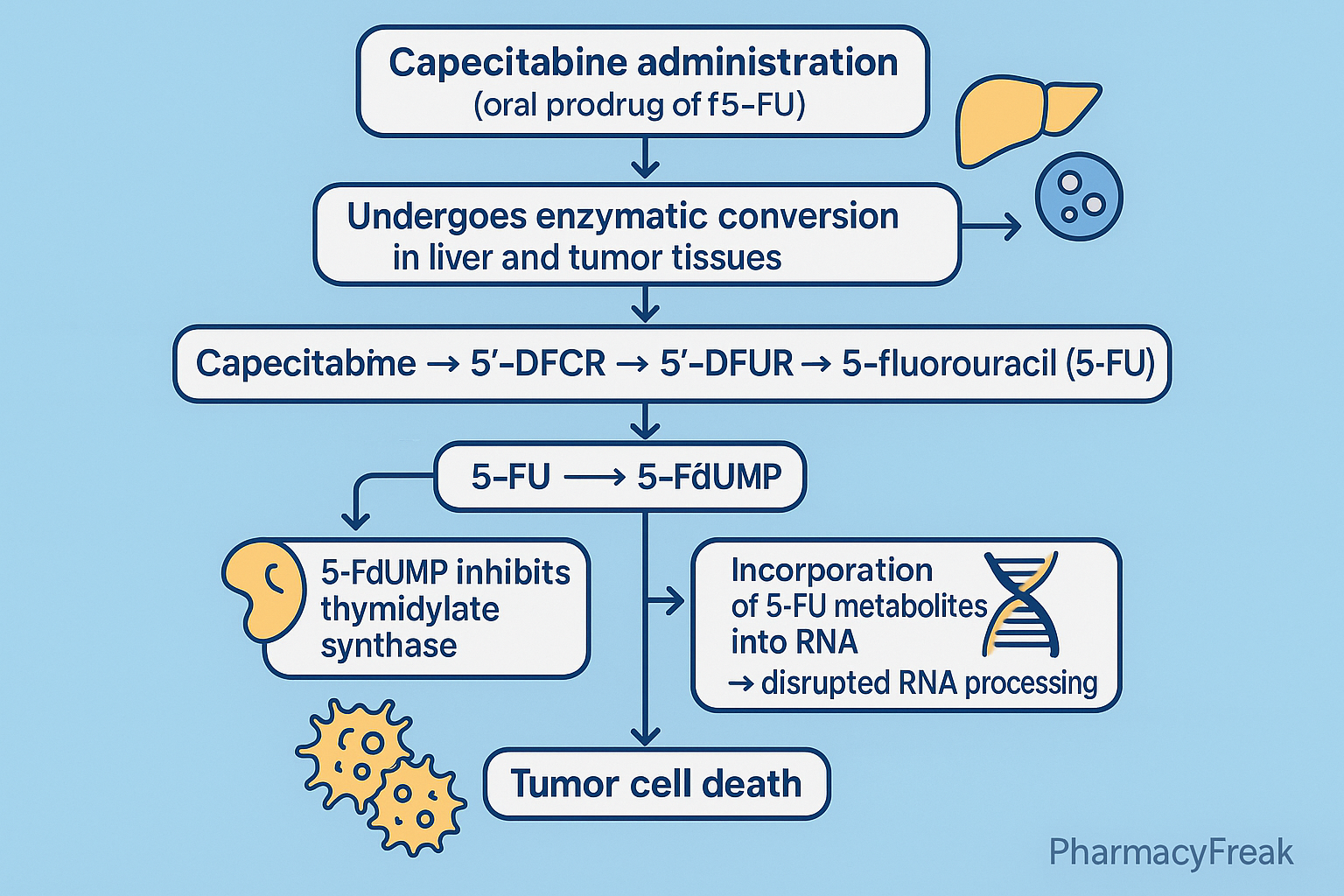
Pharmacokinetics
- Route: Oral (high bioavailability)
- Activation: Liver + tumor-specific enzymes
- Peak levels: 1.5–2 hours
- Half-life: 45–60 minutes
- Metabolism: Hepatic + tumor enzymes
- Excretion: Renal (mainly)
Clinical Uses
- Metastatic and adjuvant colorectal cancer
- Metastatic breast cancer
- Gastric cancer
- Pancreatic cancer (combinations)
- Off-label: cholangiocarcinoma, neuroendocrine tumors
Adverse Effects
- Hand-foot syndrome (erythrodysesthesia)
- Diarrhea
- Stomatitis
- Myelosuppression
- Nausea/vomiting
- Fatigue
- Hyperbilirubinemia
- Cardiovascular toxicity (rare but serious)
DPD deficiency can lead to severe toxicity and must be screened when indicated.
Contraindications
- Severe renal impairment
- Pregnancy
- Dihydropyrimidine dehydrogenase (DPD) deficiency
- Known hypersensitivity to fluoropyrimidines
Comparative Analysis
| Feature | Capecitabine | 5-Fluorouracil (IV) | Tegafur |
|---|---|---|---|
| Route | Oral | IV | Oral |
| Tumor selectivity | High | Moderate | Moderate |
| Enzyme activation | Thymidine phosphorylase | Direct drug | CYP-mediated |
| Hand-foot syndrome | Higher | Moderate | Moderate |
| Convenience | High | Low | High |
MCQs
1. Capecitabine is converted into its active form mainly by:
a) Thymidylate synthase
b) Thymidine phosphorylase
c) Dihydropyrimidine dehydrogenase
d) Cytochrome P450
Answer: b) Thymidine phosphorylase
2. The principal cytotoxic mechanism of capecitabine involves:
a) DNA alkylation
b) Protein synthesis inhibition
c) Thymidylate synthase inhibition
d) Microtubule stabilization
Answer: c) Thymidylate synthase inhibition
3. A hallmark adverse effect of capecitabine is:
a) Nephrotoxicity
b) Hand-foot syndrome
c) Cardiomyopathy
d) Hepatitis
Answer: b) Hand-foot syndrome
4. Capecitabine primarily arrests the cell cycle in:
a) G1 phase
b) S phase
c) G2 phase
d) M phase
Answer: b) S phase
5. Severe toxicity occurs in patients with deficiency of:
a) COMT
b) GST
c) DPD
d) MAO
Answer: c) DPD
FAQs
Q1. Is capecitabine the same as 5-fluorouracil?
No—capecitabine is an oral prodrug that becomes 5-FU inside tumor cells.
Q2. Why is capecitabine more tumor-selective?
Tumors have higher thymidine phosphorylase, activating more 5-FU locally.
Q3. Can capecitabine be used with radiation therapy?
Yes—often used as a radiosensitizer in GI cancers.
Q4. Why does hand-foot syndrome occur?
Due to high accumulation of metabolites in skin capillaries.
Q5. How long does capecitabine take to work?
Clinical effects usually appear within 6–8 weeks.
References
Goodman & Gilman’s Pharmacological Basis of Therapeutics
https://accesspharmacy.mhmedical.com/book.aspx?bookid=2189
Katzung: Basic and Clinical Pharmacology
https://accessmedicine.mhmedical.com/book.aspx?bookid=2464
Tripathi: Essentials of Medical Pharmacology
https://jaypeebrothers.com/
Harrison’s Principles of Internal Medicine
https://accessmedicine.mhmedical.com/book.aspx?bookid=2129
Related Internal Links

I am pursuing MBA in pharmaceutical management from NIPER Hyderabad with a strong academic record and proven success in national-level pharmacy entrance exams. I secured AIR 61 in NIPER 2024 (MS/M.Pharm) and AIR 27 in NIPER MBA, along with AIR 147 in GPAT 2024 and AIR 907 in GPAT 2023. I also achieved AIR 6 in AIIMS CRE-2025 for Drug Store Keeper and was selected as a Pharmacist (AIR 61) for ESIC. Additionally, I was the Runner-Up in Round 2 of the EY Case Study Competition.
At PharmacyFreak.com, I aim to guide future pharmacists through expert content, exam strategies, and insightful resources based on real experience and academic excellence.
Mail- harsh@pharmacyfreak.com
