Table of Contents
Introduction
Bleomycin is a cytotoxic glycopeptide antibiotic used as an antineoplastic agent, especially in the treatment of Hodgkin lymphoma, testicular cancer, squamous cell carcinomas, and germ cell tumors. It is unique among chemotherapeutic agents because it causes minimal myelosuppression but is strongly associated with pulmonary toxicity.
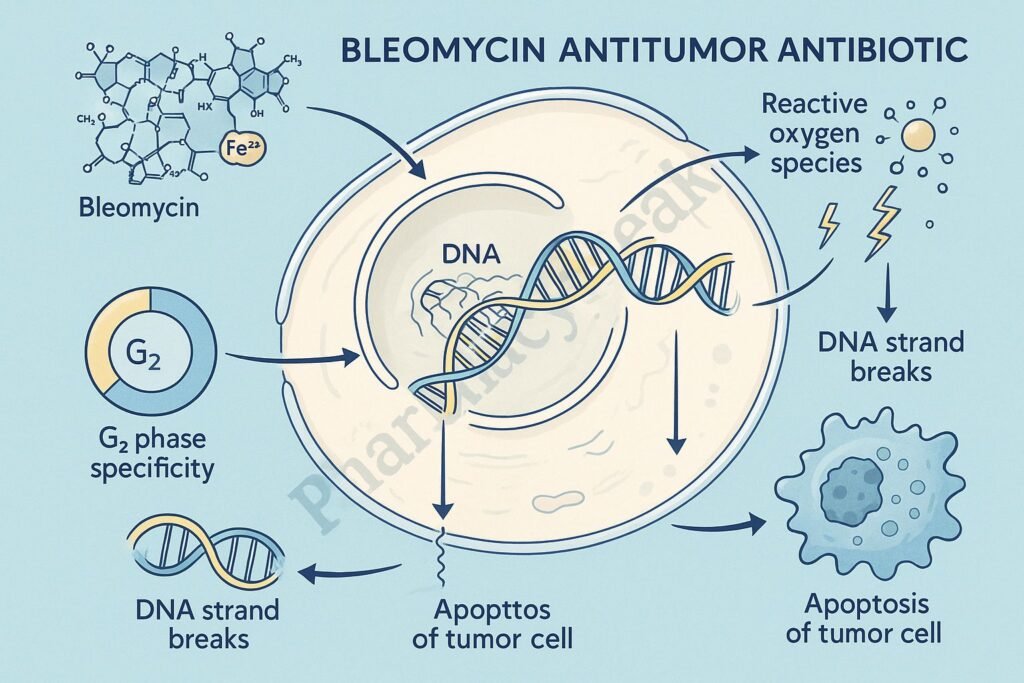
Bleomycin acts primarily during the G2 phase of the cell cycle and is highly effective due to its ability to induce DNA strand breaks through free radical formation.
Mechanism of Action (Step-wise)
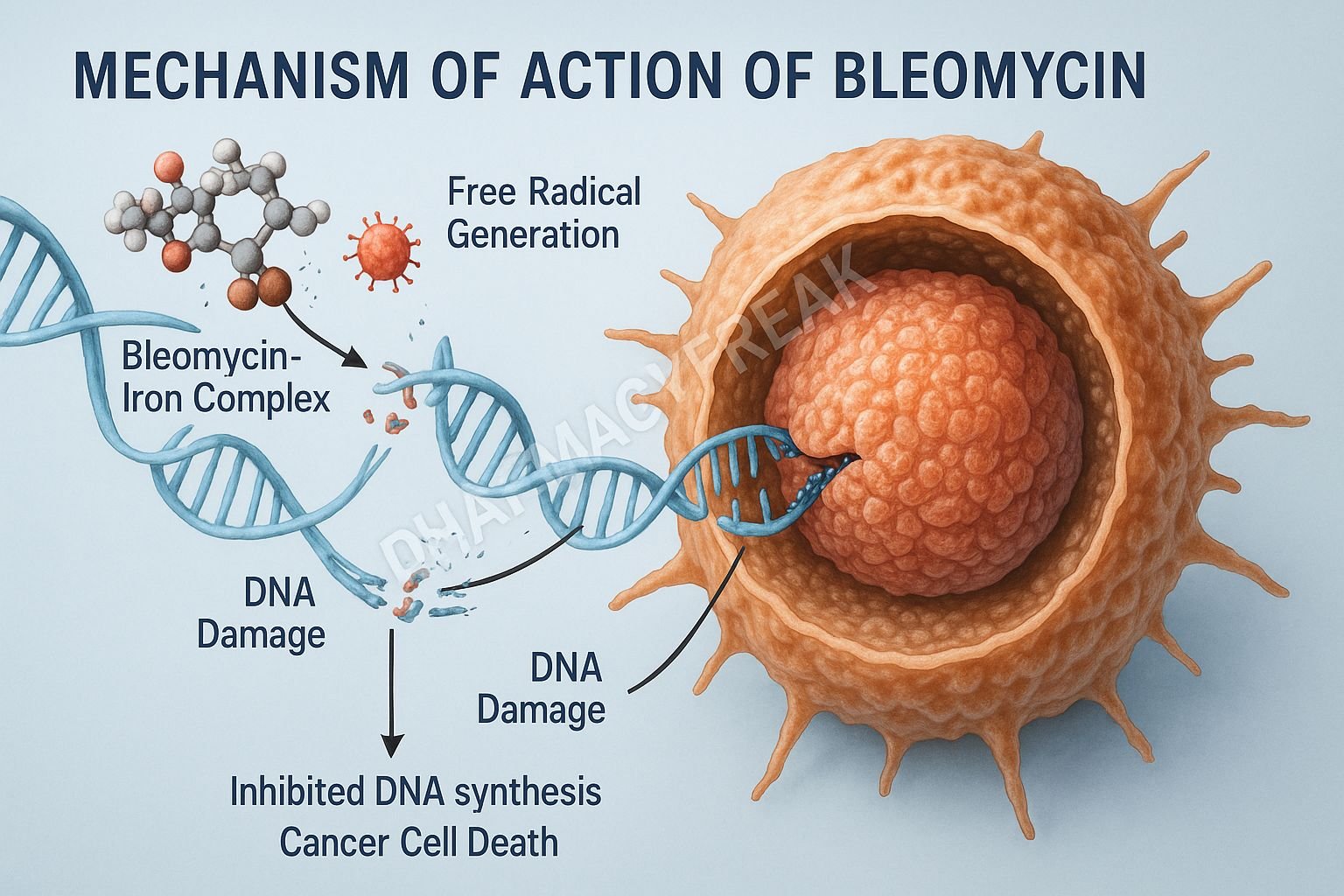
1. DNA Binding and Complex Formation
Bleomycin binds to DNA through:
- Intercalation
- Chelation of metal ions (Fe²⁺)
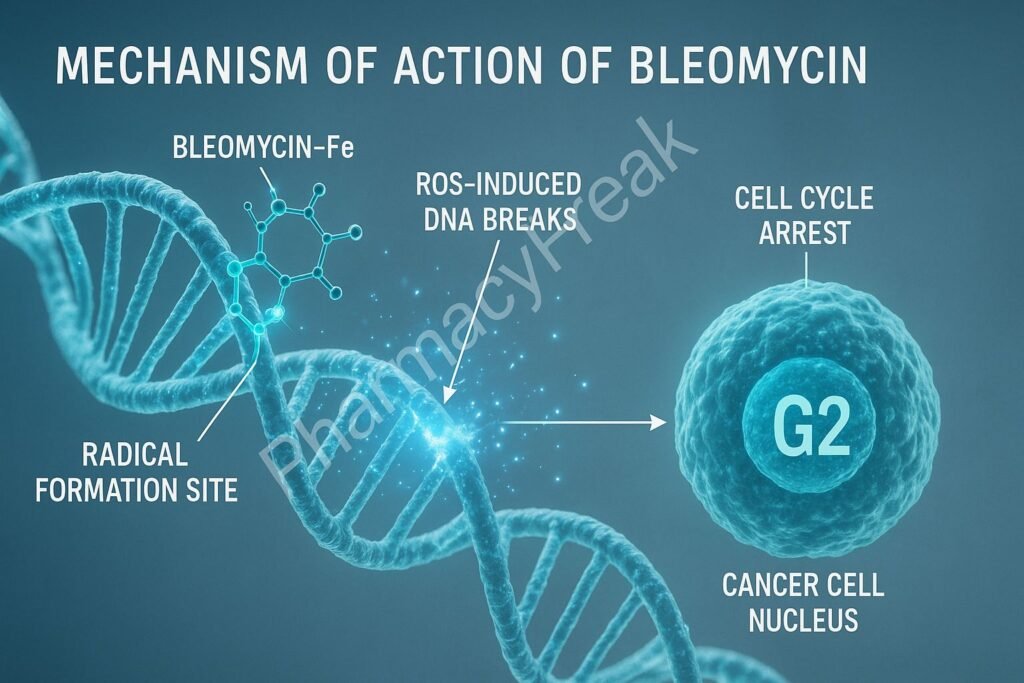
This forms a bleomycin–Fe²⁺–DNA complex, setting the stage for free radical generation.
2. Free Radical Formation (Primary Mechanism)
In the presence of oxygen, the bleomycin–Fe²⁺ complex undergoes oxidation to Fe³⁺ while generating reactive oxygen species (ROS) including:
- Superoxide
- Hydroxyl radicals
- Free radicals
These ROS directly attack DNA.
3. DNA Strand Breaks
Bleomycin causes:
- Single-strand breaks
- Double-strand breaks
These breaks result in:
- Inhibition of DNA synthesis
- Cell cycle arrest (G2 phase)
- Apoptosis of rapidly dividing cancer cells
4. Inhibition of DNA, RNA, and Protein Synthesis
Although DNA breakage is the main effect, bleomycin also:
- Impairs RNA synthesis
- Reduces protein synthesis
But these effects are secondary.
5. Selective Toxicity
Tissues with low levels of bleomycin hydrolase (the enzyme that inactivates bleomycin) are more susceptible:
- Lungs
- Skin
This explains the drug’s characteristic toxicities.
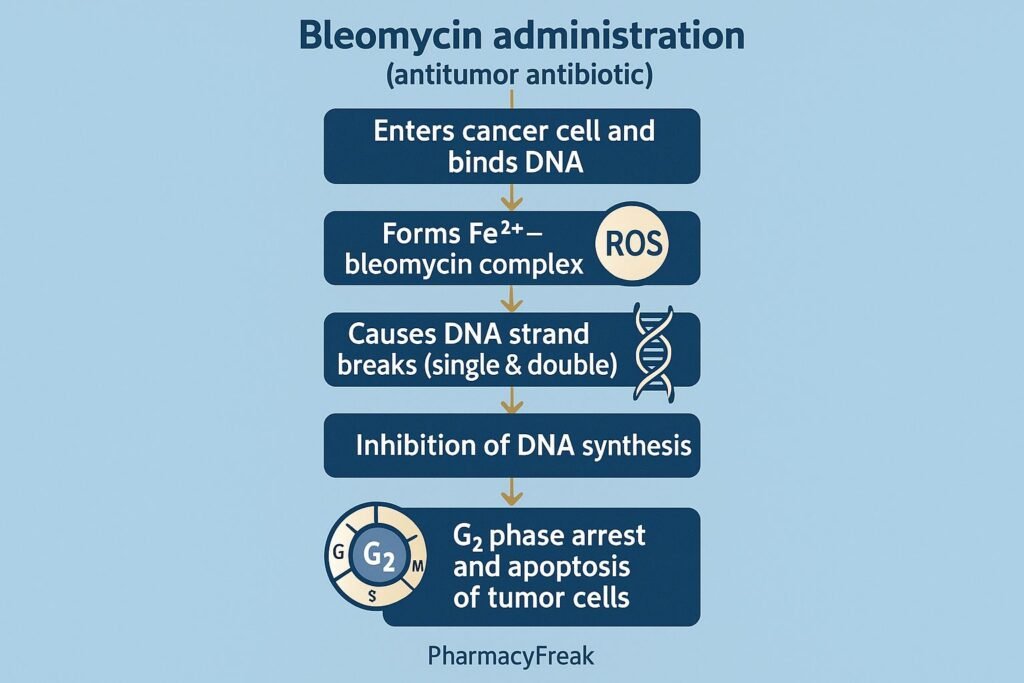
Summary of Mechanism
| Mechanism | Effect |
|---|---|
| DNA binding | Formation of bleomycin–Fe complex |
| ROS generation | DNA damage |
| Strand breaks | G2 arrest, apoptosis |
| RNA/protein inhibition | Secondary cytotoxic effects |
Pharmacokinetics
- Route: IV, IM, or subcutaneous
- Distribution: Poor CNS penetration
- Metabolism: Inactivated by bleomycin hydrolase
- Excretion: Mainly renal
- Half-life: 2–4 hours (longer with renal impairment)
Clinical Uses
- Hodgkin lymphoma (ABVD regimen)
- Non-Hodgkin lymphoma
- Testicular cancer
- Ovarian germ cell tumors
- Squamous cell carcinoma of skin, head, neck, cervix
- Malignant pleural effusion (intracavitary)
Adverse Effects
1. Pulmonary Toxicity (Most Serious)
- Pneumonitis
- Pulmonary fibrosis
- Dose-limiting toxicity
Risk factors:
- Age > 70
- Renal impairment
- High cumulative dose (>400 units)
- Prior chest radiation
- Oxygen therapy
2. Skin Toxicity
- Hyperpigmentation
- Erythema
- Ulceration
- Alopecia
- Nail changes
3. Other Adverse Effects
- Fever
- Chills
- Mucositis
- Anaphylaxis (rare)
Contraindications
- Severe pulmonary disease
- Pregnancy
- Caution in renal failure
Comparative Analysis
| Feature | Bleomycin | Doxorubicin | Cyclophosphamide |
|---|---|---|---|
| Mechanism | DNA free radical damage | Topoisomerase II inhibition | Alkylation |
| Bone marrow suppression | Minimal | Severe | Moderate–severe |
| Major toxicity | Pulmonary fibrosis | Cardiotoxicity | Hemorrhagic cystitis |
| Cell cycle | G2 phase | S phase | Cell cycle–nonspecific |
MCQs
1. Bleomycin primarily causes:
a) Microtubule inhibition
b) DNA strand breaks via free radicals
c) Topoisomerase inhibition
d) Alkylation
Answer: b) DNA strand breaks via free radicals
2. Bleomycin acts mainly in which phase of the cell cycle?
a) S
b) M
c) G2
d) G1
Answer: c) G2
3. The major dose-limiting toxicity of bleomycin is:
a) Bone marrow suppression
b) Nephrotoxicity
c) Pulmonary fibrosis
d) Cardiotoxicity
Answer: c) Pulmonary fibrosis
4. Bleomycin is inactivated by:
a) Cytochrome P450
b) Bleomycin hydrolase
c) Glutathione S-transferase
d) Aldehyde dehydrogenase
Answer: b) Bleomycin hydrolase
5. Bleomycin generates free radicals by complexing with:
a) Copper
b) Magnesium
c) Iron
d) Zinc
Answer: c) Iron
FAQs
Q1. Why does bleomycin cause lung toxicity?
Lung tissue has low levels of bleomycin hydrolase, resulting in drug accumulation and oxidative damage.
Q2. Does bleomycin cause bone marrow suppression?
Minimal—this is why it is favored in combination regimens like ABVD.
Q3. Can oxygen therapy worsen toxicity?
Yes—oxygen increases oxidative stress and risk of pulmonary fibrosis.
Q4. How is bleomycin given?
Usually via IV injection; can also be used intrapleurally for malignant effusions.
Q5. What cancer regimen commonly includes bleomycin?
ABVD for Hodgkin lymphoma.
References
Goodman & Gilman’s Pharmacological Basis of Therapeutics
https://accesspharmacy.mhmedical.com/book.aspx?bookid=2189
Katzung: Basic & Clinical Pharmacology
https://accessmedicine.mhmedical.com/book.aspx?bookid=2464
Tripathi: Essentials of Medical Pharmacology
https://jaypeebrothers.com/
Harrison’s Principles of Internal Medicine
https://accessmedicine.mhmedical.com/book.aspx?bookid=2129

I am pursuing MBA in pharmaceutical management from NIPER Hyderabad with a strong academic record and proven success in national-level pharmacy entrance exams. I secured AIR 61 in NIPER 2024 (MS/M.Pharm) and AIR 27 in NIPER MBA, along with AIR 147 in GPAT 2024 and AIR 907 in GPAT 2023. I also achieved AIR 6 in AIIMS CRE-2025 for Drug Store Keeper and was selected as a Pharmacist (AIR 61) for ESIC. Additionally, I was the Runner-Up in Round 2 of the EY Case Study Competition.
At PharmacyFreak.com, I aim to guide future pharmacists through expert content, exam strategies, and insightful resources based on real experience and academic excellence.
Mail- harsh@pharmacyfreak.com