Table of Contents
1. Identification
Summary
Testosterone is an endogenous androgen used therapeutically for male hypogonadism (primary or hypogonadotropic) and certain other indications. It acts via androgen receptor (AR) agonism and through metabolites dihydrotestosterone (5-α-reduction) and estradiol (aromatization).
Brand Names
Multiple by formulation (e.g., transdermal gels/patches, IM esters, pellets; region-dependent)
Name
Testosterone
Background
Principal male sex hormone; administered as base (transdermal, buccal, nasal) or as long-acting esters (e.g., cypionate, enanthate, undecanoate) for depot delivery.
Modality
Small molecule
Groups
Approved; prescription/controlled (jurisdiction-specific)
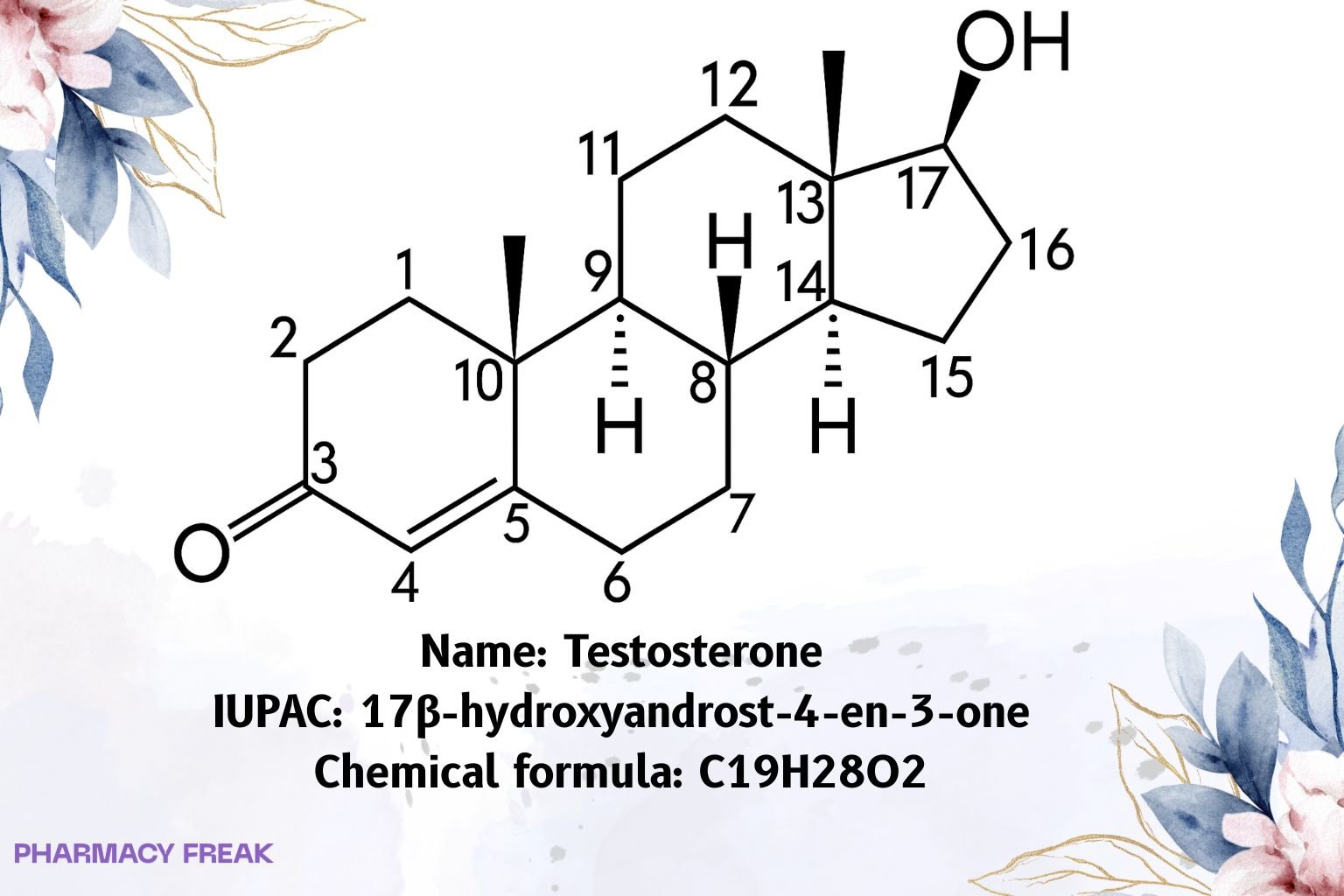
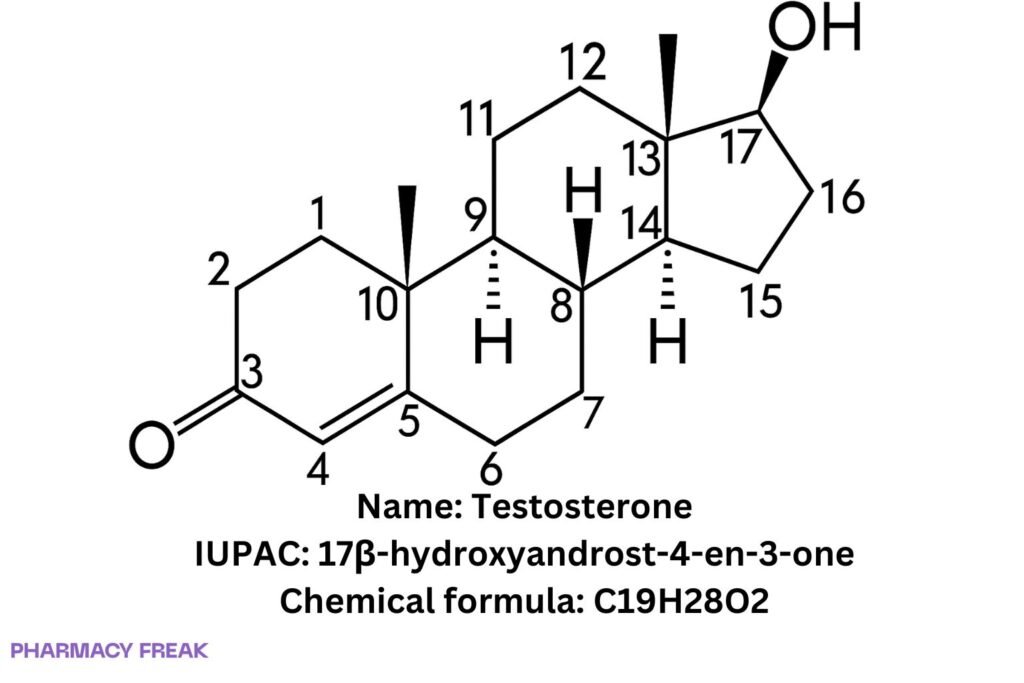
Structure
Weight
~288.42 g/mol (free base)
Chemical Formula
C₁₉H₂₈O₂
Synonyms
17β-hydroxyandrost-4-en-3-one; Androst-4-en-17β-ol-3-one
External IDs
CAS: 58-22-0; PubChem CID: 6013; UNII: 3XMK78S47O; ATC: G03BA03
2. Pharmacology
Indication
Male hypogonadism (primary or hypogonadotropic); delayed puberty in males (selected cases); palliative use in some inoperable breast cancers (product-/region-specific).
Associated Conditions
Hypogonadism symptoms/signs (low serum T confirmed), osteoporosis/low bone mass related to androgen deficiency; gender-affirming hormone therapy (clinical practice, off-label in some regions).
Associated Therapies
IM depot esters (cypionate, enanthate, undecanoate), transdermal gel/patch, nasal and buccal formulations, subcutaneous pellets.
Contraindications & Blackbox Warnings
Contraindicated in men with breast or known/suspected prostate cancer, women who are or may become pregnant, and in hypersensitivity to components.
Major warnings by product: erythrocytosis/polycythemia, worsening BPH/urinary symptoms, potential blood pressure increase (some formulations), lipid changes, edema, sleep apnea, thromboembolic events; pulmonary oil microembolism/anaphylaxis warning with certain undecanoate IM products (REMS in some markets).
Pharmacodynamics
Binds AR → gene transcription driving virilization, anabolism, and erythropoiesis; peripheral conversion to DHT (potent AR agonist) and estradiol (bone/brain effects).
Mechanism of action
AR agonism; 5-α-reductase → DHT; aromatase → estradiol; broad tissue-specific actions via receptor distribution/co-regulators.
Absorption
Route-dependent:
• Transdermal: systemic absorption from gel/patch; steady state in days.
• IM esters: slow release from oil depot.
• Oral undecanoate: lymphatic uptake; fat-dependent absorption.
• Oral base: poor (extensive first-pass metabolism).
Volume of distribution
Large, with extensive tissue distribution (steroid class).
Protein binding
High (~98%): to SHBG and albumin; ~1–2% free fraction.
Metabolism
Hepatic: 5-α-reduction (→ DHT), aromatization (→ estradiol), and phase II conjugation (e.g., UGT2B17 glucuronidation) to inactive metabolites.
Route of elimination
Primarily urine (~90%) as glucuronide/sulfate conjugates; feces ~6% (metabolites).
Half-life
Free testosterone in plasma: variable, ~10–100 min (short).
Depot esters (e.g., cypionate): apparent t½ ~8 days IM; others formulation-dependent.
Clearance
Predominantly hepatic metabolic clearance; conjugate renal excretion.
Adverse Effects
Acne, seborrhea, alopecia (androgenic), gynecomastia (aromatization), mood/libido changes, erythrocytosis (↑Hct), edema, hypertension (some products), fertility suppression (↓spermatogenesis), injection/dermal site reactions; rare serious thromboembolic and cardiovascular events (risk signals product-/population-dependent).
Toxicity
Overexposure/abuse (anabolic-androgenic steroid misuse): hepatic strain (notably with 17-α-alkylated agents, less with testosterone), psychiatric effects, dyslipidemia, endocrine suppression. Supportive management; taper under medical care.
Pathways
AR signaling; 5-α-reduction and aromatase pathways; conjugation and urinary excretion of 17-ketosteroid derivatives.
Pharmacogenomic Effects/ADRs
UGT2B17 deletion polymorphism affects urinary excretion profiles (forensic/analytical relevance). SRD5A2 variants modulate DHT formation; AR CAG repeat length influences sensitivity (research/clinical nuance).
3. Interactions
Drug Interactions
Oral anticoagulants (e.g., warfarin): potentiation (↑INR) reported; monitor.
Insulin/antidiabetics: dose adjustments may be needed.
Corticosteroids: additive edema/fluid retention.
Drugs altering SHBG (e.g., thyroxine) can shift free T fraction.
Food Interactions
Oral undecanoate: high-fat meal increases absorption; follow product labeling.
4. Categories
ATC Codes
G03BA03 (androgens)
Drug Categories
Androgen; Anabolic steroid; Endogenous hormone; Small molecule
Chemical Taxonomy
C19 androstane steroid with 3-keto and 17β-hydroxy groups; Δ⁴-ene.
Affected organisms
Humans (therapeutic use)
5. Chemical Identifiers
UNII
3XMK78S47O
CAS number
58-22-0
InChI Key
MUMGGOZAMZWBJJ-DYKIIFRCSA-N
InChI
InChI=1S/C19H28O2/c1-18-9-7-13(20)11-12(18)3-4-14-15-5-6-17(21)19(15,2)10-8-16(14)18/h11,14-17,21H,3-10H2,1-2H3/t14-,15-,16-,17-,18-,19-/m0/s1
IUPAC Name
(8R,9S,10R,13S,14S,17S)-17-hydroxy-10,13-dimethyl-1,2,6,7,8,9,11,12,14,15,16,17-dodecahydrocyclopentaaaaphenanthren-3-one
(Short: 17β-hydroxyandrost-4-en-3-one)
SMILES
O=C1C=C2C@(CC1)[C@H]3CC[C@]4(C)C@HO
6. References
PubChem record for Testosterone (CID 6013) — identifiers, formula, synonyms. PubChem
CAS Common Chemistry — CAS 58-22-0, InChI, InChIKey MUMGGOZAMZWBJJ-DYKIIFRCSA-N, canonical SMILES. commonchemistry.cas.org
precisionFDA/UNII — UNII 3XMK78S47O (Testosterone). precisionFDA
WHOCC ATC/DDD Index — G03BA03 classification. ATCDDD
DailyMed (testosterone cypionate) — IM depot t½ ~8 days, ~90% urine conjugates / ~6% feces, hepatic inactivation. DailyMed+1
DailyMed (testosterone gel) — plasma half-life range ~10–100 min for free testosterone reported. DailyMed

I am pursuing MBA in pharmaceutical management from NIPER Hyderabad with a strong academic record and proven success in national-level pharmacy entrance exams. I secured AIR 61 in NIPER 2024 (MS/M.Pharm) and AIR 27 in NIPER MBA, along with AIR 147 in GPAT 2024 and AIR 907 in GPAT 2023. I also achieved AIR 6 in AIIMS CRE-2025 for Drug Store Keeper and was selected as a Pharmacist (AIR 61) for ESIC. Additionally, I was the Runner-Up in Round 2 of the EY Case Study Competition.
At PharmacyFreak.com, I aim to guide future pharmacists through expert content, exam strategies, and insightful resources based on real experience and academic excellence.
Mail- harsh@pharmacyfreak.com