Table of Contents
Introduction
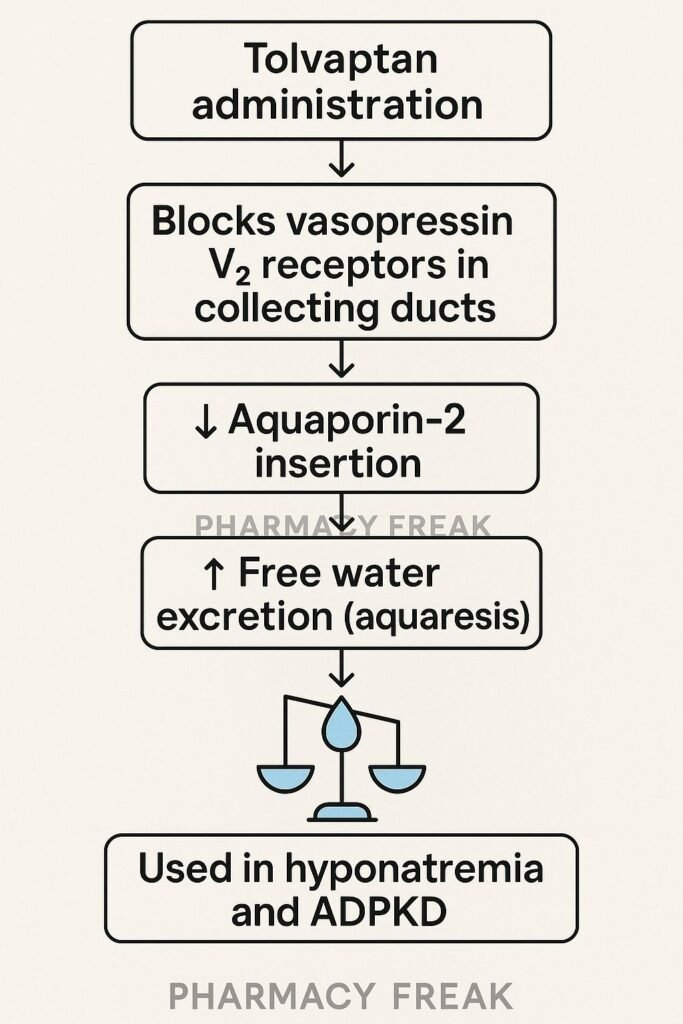
Tolvaptan is an oral vasopressin V₂ receptor antagonist, approved for treating hyponatremia due to SIADH, heart failure, or cirrhosis, and for slowing disease progression in autosomal dominant polycystic kidney disease (ADPKD). It increases free water excretion (aquaretic effect) without significant electrolyte loss.
Step-by-Step Mechanism of Action
- Selective V₂ receptor antagonism
Tolvaptan binds to and blocks vasopressin V₂ receptors located in the collecting ducts of the kidneys. - Inhibition of aquaporin-2 channel insertion
This prevents vasopressin-induced insertion of aquaporin-2 water channels into the apical membrane. - Free water diuresis (aquaresis)
With fewer water channels, water reabsorption decreases, leading to excretion of dilute urine. - Correction of serum sodium
Loss of free water raises serum sodium concentration, correcting hyponatremia. - Reduced cAMP levels in ADPKD
In polycystic kidneys, V₂ receptor inhibition reduces intracellular cAMP, decreasing cyst cell proliferation and fluid secretion.
Pharmacokinetic Parameters
| Parameter | Value |
|---|---|
| Route | Oral |
| Bioavailability | ~40% |
| Time to Peak (Tmax) | 2–4 hours |
| Protein Binding | ~99% |
| Metabolism | Hepatic (CYP3A4) |
| Half-life | 8–12 hours |
| Excretion | Mainly hepatic |
Clinical Uses
- Euvolemic and hypervolemic hyponatremia (e.g., SIADH, CHF, cirrhosis)
- Autosomal dominant polycystic kidney disease (ADPKD)
Adverse Effects
- Thirst, dry mouth
- Polyuria and nocturia
- Risk of overly rapid correction of sodium
- Hepatotoxicity (especially in long-term use for ADPKD)
- Headache, nausea
Comparative Analysis
| Drug | Target | Effect | Key Use |
|---|---|---|---|
| Tolvaptan | V₂ receptor | Aquaretic diuresis | Hyponatremia, ADPKD |
| Conivaptan | V₁/V₂ receptors | Mixed vasodilation & aquaresis | ICU hyponatremia |
| Furosemide | NKCC2 in TAL | Natriuretic diuresis | Edema, CHF, hypertension |
MCQs
- Tolvaptan primarily blocks:
a) V₁ receptor b) V₂ receptor c) Aquaporin-1 d) Sodium channel
Answer: b) V₂ receptor - Which type of diuresis does tolvaptan cause?
a) Natriuresis b) Osmotic diuresis c) Aquaretic diuresis d) Glucosuria
Answer: c) Aquaretic diuresis - Tolvaptan raises serum sodium by:
a) Increasing sodium intake b) Inhibiting aldosterone c) Reducing free water reabsorption d) Promoting potassium excretion
Answer: c) Reducing free water reabsorption - The aquaretic effect is due to inhibition of:
a) Aquaporin-3 b) Sodium channel c) Aquaporin-2 insertion d) Renin release
Answer: c) Aquaporin-2 insertion - In ADPKD, tolvaptan reduces:
a) Urine osmolality b) Serum sodium c) cAMP levels d) BUN
Answer: c) cAMP levels - Tolvaptan metabolism primarily involves:
a) CYP2D6 b) CYP3A4 c) UGT1A1 d) CYP2C9
Answer: b) CYP3A4 - Which condition is a contraindication?
a) Hypertension b) SIADH c) Hypovolemic hyponatremia d) Polycystic liver disease
Answer: c) Hypovolemic hyponatremia - Protein binding of tolvaptan is approximately:
a) 60% b) 75% c) 90% d) 99%
Answer: d) 99% - Main side effect requiring caution:
a) Bradycardia b) Liver injury c) Rash d) Hypokalemia
Answer: b) Liver injury - Time to peak plasma concentration:
a) 30 minutes b) 1 hour c) 2–4 hours d) 8 hours
Answer: c) 2–4 hours
FAQs
1. Can tolvaptan be used long-term?
Only in ADPKD under REMS monitoring. For hyponatremia, duration is limited to 30 days.
2. How quickly does it increase serum sodium?
Effect begins within hours; sodium rise must be monitored to prevent overly rapid correction.
3. Why is fluid restriction not advised initially?
Because the drug promotes free water loss; restricting fluids too early may increase sodium too quickly.
4. Is it safe in liver disease?
Use with caution—tolvaptan can be hepatotoxic and requires monitoring.
5. Is tolvaptan a loop diuretic?
No—it is a selective aquaretic that conserves electrolytes while increasing water excretion.
References
- DrugBank: Tolvaptan
- FDA Label: Tolvaptan (Samsca)
- PubMed Review: Tolvaptan in ADPKD
- StatPearls: Tolvaptan Pharmacology

I am pursuing MBA in pharmaceutical management from NIPER Hyderabad with a strong academic record and proven success in national-level pharmacy entrance exams. I secured AIR 61 in NIPER 2024 (MS/M.Pharm) and AIR 27 in NIPER MBA, along with AIR 147 in GPAT 2024 and AIR 907 in GPAT 2023. I also achieved AIR 6 in AIIMS CRE-2025 for Drug Store Keeper and was selected as a Pharmacist (AIR 61) for ESIC. Additionally, I was the Runner-Up in Round 2 of the EY Case Study Competition.
At PharmacyFreak.com, I aim to guide future pharmacists through expert content, exam strategies, and insightful resources based on real experience and academic excellence.
Mail- harsh@pharmacyfreak.com
