Table of Contents
Introduction
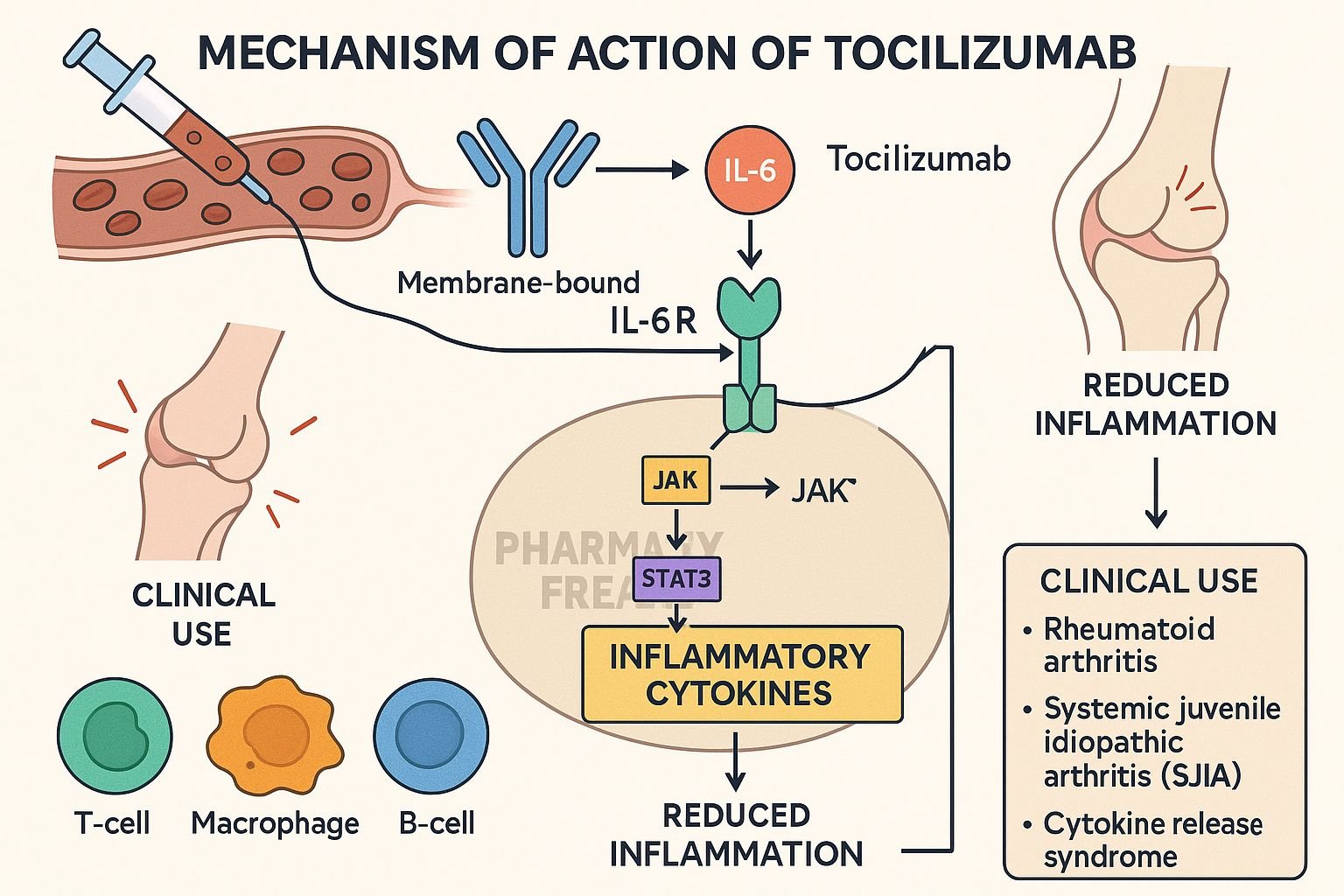
Tocilizumab is a humanized monoclonal antibody targeting the interleukin-6 receptor (IL-6R). It is approved for rheumatoid arthritis (RA), juvenile idiopathic arthritis (JIA), giant cell arteritis (GCA), Castleman disease, cytokine release syndrome (CRS), and spondyloarthritis-associated uveitis. By blocking IL-6 signaling, it dampens inflammation in various autoimmune and inflammatory diseases.
Step-by-Step Mechanism of Action
- Binding to IL-6 receptors
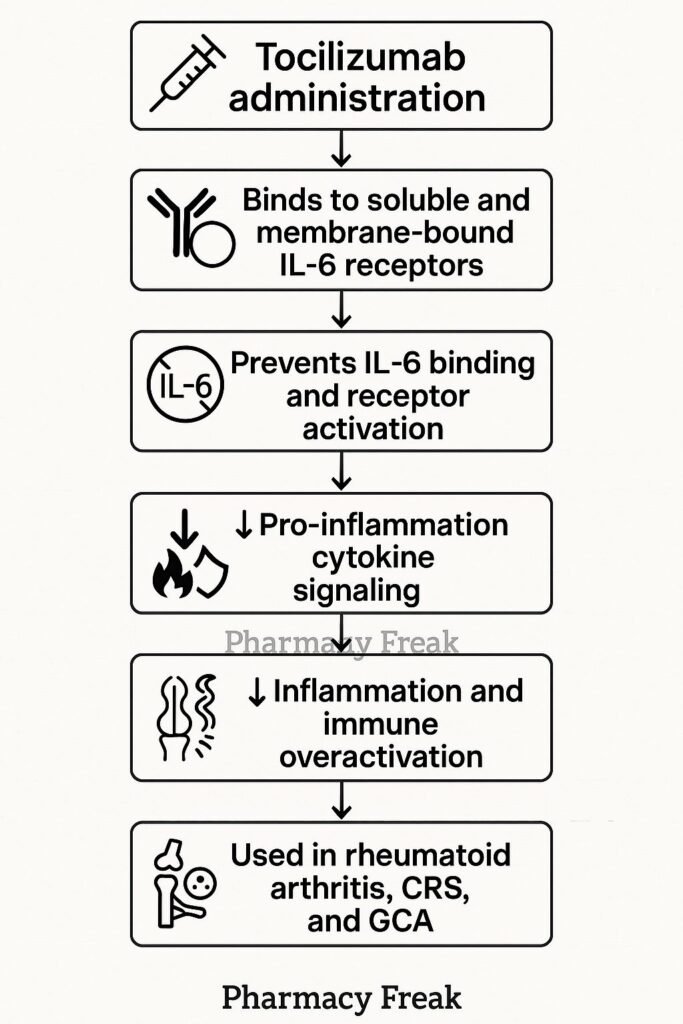
Tocilizumab binds both membrane-bound and soluble IL‑6 receptors (mIL‑6R and sIL‑6R), preventing IL‑6 from initiating signaling. - Prevention of receptor complex formation
This disrupts the formation of the IL‑6/IL‑6R/gp130 complex, which is necessary to activate downstream pathways. - Inhibition of JAK‑STAT signaling
Without receptor complex activation, JAKs are not activated, and STAT transcription factors are not phosphorylated. - Suppression of pro-inflammatory gene expression
Blocking STAT activation reduces synthesis of CRP, fibrinogen, hepcidin, and other inflammatory proteins. - Reduction of inflammation and immune cell activation
The net effect is decreased acute-phase responses, joint inflammation, vascular inflammation, and modulation of T‑cell differentiation.
Pharmacokinetic Parameters
| Parameter | Value |
|---|---|
| Route | Intravenous (IV) every 4 weeks or Subcutaneous weekly |
| Distribution | Extracellular fluid; limited tissue penetration |
| Half-life | ~8–14 days (dose-dependent) |
| Metabolism | Proteolytic degradation |
| Excretion | Non-renal pathways; degraded into peptides |
Clinical Uses
- Moderate to severe rheumatoid arthritis (with or without other DMARDs)
- Systemic and polyarticular juvenile idiopathic arthritis
- Giant cell arteritis
- Cytokine release syndrome post CAR-T therapy
- Castleman disease, spondyloarthritis-associated uveitis
Adverse Effects
- Increased risk of serious infections (e.g., TB, pneumonia)
- Elevated liver enzymes and lipid levels
- Infusion or injection site reactions
- Cytopenias (e.g., neutropenia)
- Gastrointestinal perforation risk in patients with GI disease
Comparative Analysis
| Agent | Target | Dosing Frequency | Indications |
|---|---|---|---|
| Tocilizumab | IL‑6 receptor (mIL‑6R/sIL‑6R) | IV every 4 weeks / SC weekly | RA, GCA, JIA, CRS, Castleman disease |
| Sarilumab | IL‑6 receptor (mIL‑6R only) | SC every 2 weeks | RA |
| Tocilizumab | TNF‑α | Varies | RA but different cytokine target |
MCQs
- Tocilizumab binds to which receptor?
a) TNF‑αR b) IL‑6R c) IL‑1R d) CD20
Answer: b) IL‑6R - It prevents IL‑6 from triggering which cascade?
a) JAK‑STAT b) MAPK only c) NF‑κB only d) TGF‑β
Answer: a) JAK‑STAT - Tocilizumab targets:
a) Only membrane IL‑6R b) Only soluble IL‑6R c) Both mIL‑6R and sIL‑6R d) IL‑6 ligand
Answer: c) Both mIL‑6R and sIL‑6R - Common lab effects include:
a) Hypolipidemia and low liver enzymes b) Elevated lipids and LFTs c) Elevated potassium d) Hypoglycemia
Answer: b) Elevated lipids and LFTs - Used to treat:
a) Type 1 diabetes b) Rheumatoid arthritis c) Epilepsy d) Migraine
Answer: b) Rheumatoid arthritis - Dosing frequency for IV administration is:
a) Daily b) Weekly c) Every 4 weeks d) Monthly
Answer: c) Every 4 weeks - Adverse effect requiring caution is:
a) GI perforation b) Auditory loss c) Hypothyroidism d) Vision loss
Answer: a) GI perforation - Compared to sarilumab, tocilizumab:
a) Targets TNF‑α b) Binds both IL‑6R forms c) Is oral d) Is IL‑1 antagonist
Answer: b) Binds both IL‑6R forms - Mechanism includes reducing acute-phase proteins like:
a) Albumin b) CRP c) Troponin d) CK-MB
Answer: b) CRP - Primary route of elimination is:
a) Renal b) Hepatic metabolism c) Proteolytic degradation d) Biliary excretion
Answer: c) Proteolytic degradation
FAQs
1. Can tocilizumab be used during cytokine release syndrome?
Yes—it is the first-line therapy to reduce IL-6 mediated inflammation in CRS.
2. Is TB screening required before initiation?
Yes—latent TB should be ruled out before starting therapy.
3. How frequently should lipids and LFTs be monitored?
Check every 4–8 weeks for the first few months, then periodically.
4. Can live vaccines be given?
No—live vaccines should be avoided during treatment and for several months after.
5. How long until clinical improvement is seen?
For RA, symptomatic improvement typically occurs within 2–4 weeks.
References

I am pursuing MBA in pharmaceutical management from NIPER Hyderabad with a strong academic record and proven success in national-level pharmacy entrance exams. I secured AIR 61 in NIPER 2024 (MS/M.Pharm) and AIR 27 in NIPER MBA, along with AIR 147 in GPAT 2024 and AIR 907 in GPAT 2023. I also achieved AIR 6 in AIIMS CRE-2025 for Drug Store Keeper and was selected as a Pharmacist (AIR 61) for ESIC. Additionally, I was the Runner-Up in Round 2 of the EY Case Study Competition.
At PharmacyFreak.com, I aim to guide future pharmacists through expert content, exam strategies, and insightful resources based on real experience and academic excellence.
Mail- harsh@pharmacyfreak.com