Table of Contents
Introduction
Pioglitazone is an oral antidiabetic agent belonging to the thiazolidinedione (TZD) class. It is used primarily in the management of type 2 diabetes mellitus by improving insulin sensitivity. Unlike insulin secretagogues, pioglitazone does not increase insulin secretion but acts on peripheral tissues to enhance insulin action. Its unique mechanism involves modulation of gene expression through nuclear receptor activation, making it beneficial in reducing insulin resistance.
Mechanism of Action (Step-wise)
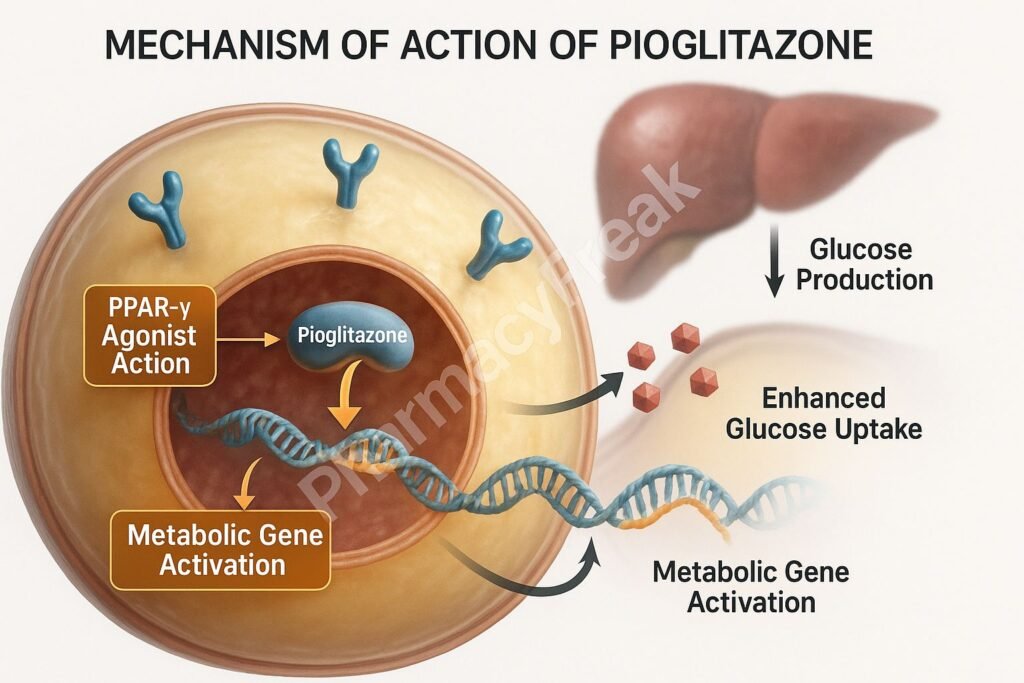
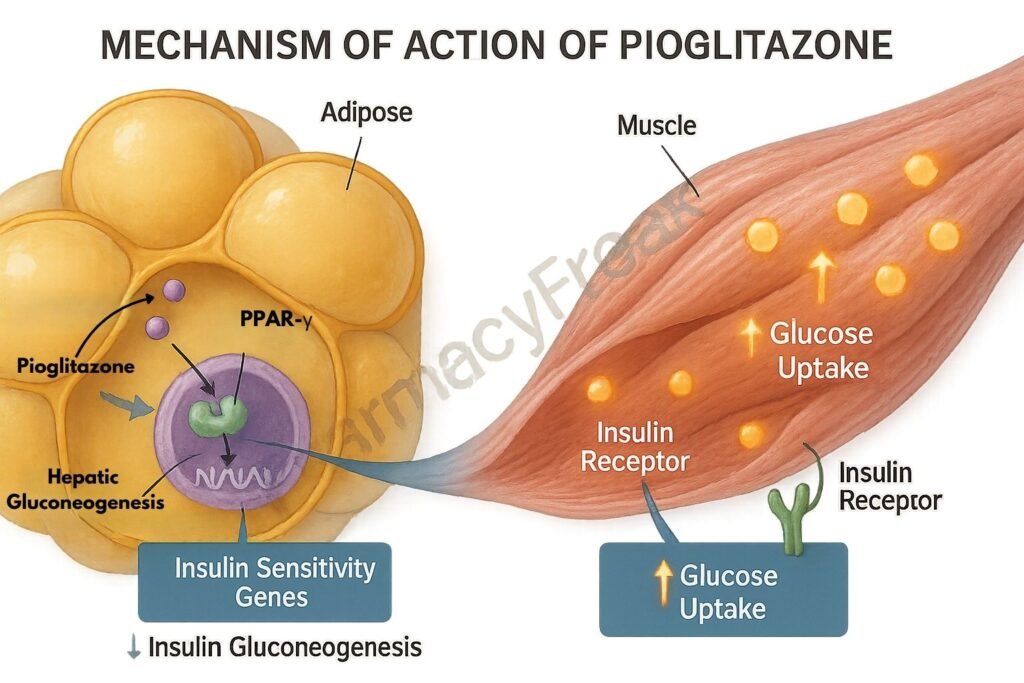
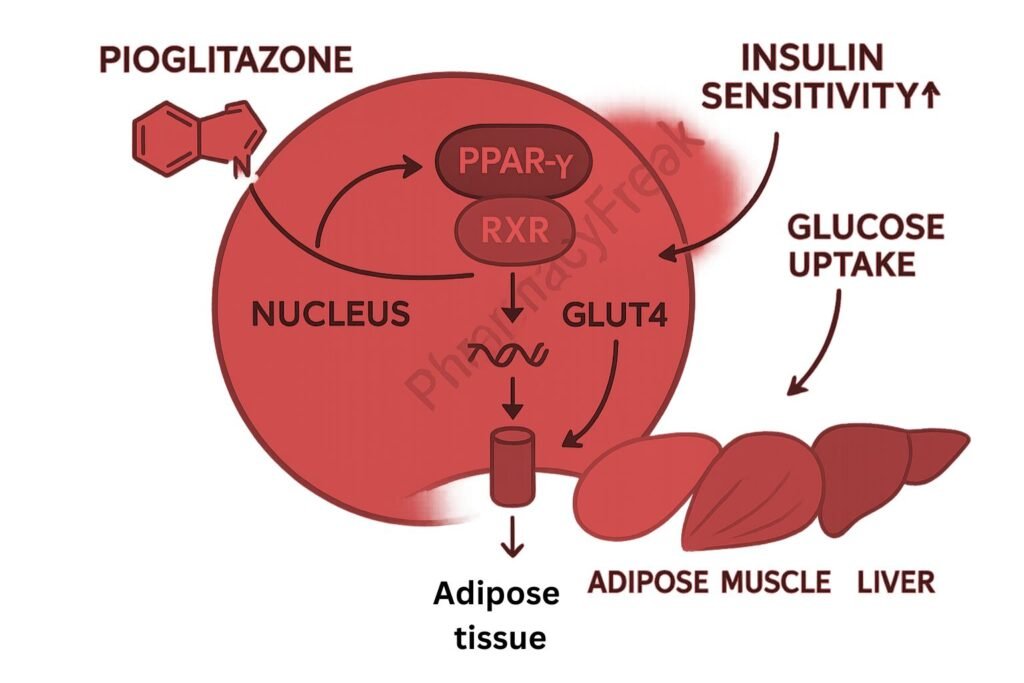
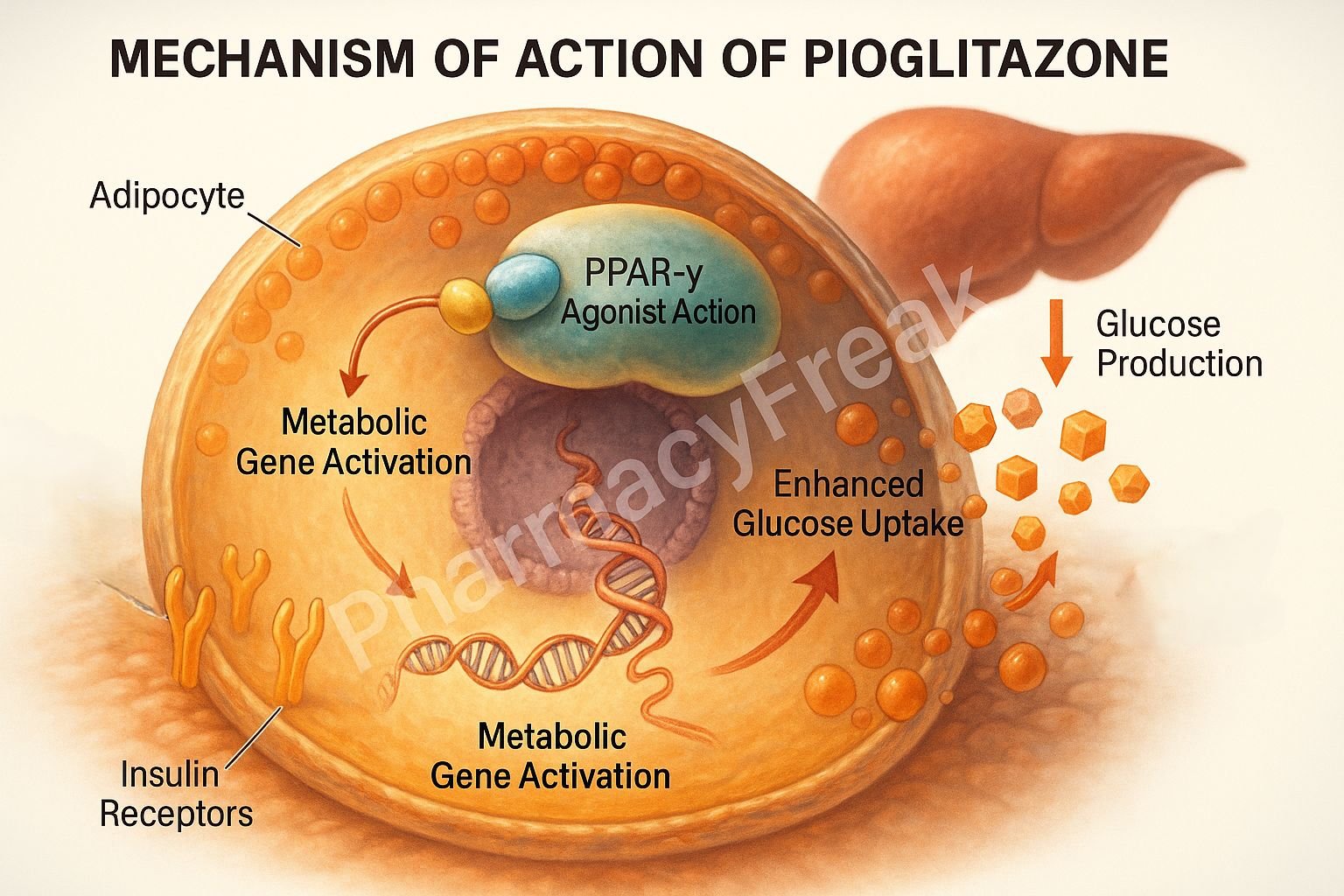
1. Activation of PPAR-γ (Peroxisome Proliferator-Activated Receptor Gamma)
Pioglitazone binds selectively to PPAR-γ, a nuclear receptor primarily found in adipose tissue, skeletal muscle, and liver.
2. Regulation of Gene Transcription
Once activated, PPAR-γ forms a heterodimer with retinoid X receptor (RXR) and binds to specific DNA sequences to modulate gene transcription.
3. Enhanced Glucose Uptake
This receptor activation increases the expression of glucose transporter type 4 (GLUT4), leading to improved glucose uptake in muscle and adipose tissue.
4. Decreased Hepatic Gluconeogenesis
Pioglitazone also reduces glucose production by the liver, helping lower fasting glucose levels.
5. Redistribution of Fat
It promotes differentiation of adipocytes and favors subcutaneous fat deposition over visceral fat, improving insulin sensitivity.
6. Anti-inflammatory Effects
PPAR-γ activation leads to reduced production of inflammatory cytokines such as TNF-α and IL-6.

Pharmacokinetics
- Route of Administration: Oral
- Bioavailability: >80%
- Time to Peak Plasma: 2 hours
- Half-Life: 3–7 hours (pioglitazone), active metabolites up to 24 hours
- Metabolism: Hepatic (CYP2C8 and CYP3A4 pathways)
- Excretion: Primarily via feces (biliary), minor renal excretion
Clinical Uses
- Type 2 diabetes mellitus (monotherapy or combination therapy)
- Insulin resistance syndrome (off-label)
- Polycystic ovary syndrome (PCOS) (off-label)
Adverse Effects
- Weight gain
- Edema and fluid retention
- Congestive heart failure (dose-related risk)
- Bone fractures in women
- Hepatotoxicity (rare)
- Bladder cancer (controversial, long-term risk)
- Contraindications: NYHA Class III/IV heart failure, active liver disease, history of bladder cancer
Comparative Analysis
| Parameter | Pioglitazone | Metformin |
|---|---|---|
| Class | Thiazolidinedione | Biguanide |
| Primary Action | Insulin sensitizer | Hepatic glucose suppression |
| Risk of Hypoglycemia | Low | Low |
| Weight Effect | Gain | Neutral or loss |
| Effect on Lipids | Improves HDL, lowers TGs | Neutral |
| Contraindications | CHF, bladder cancer | Renal failure |
Multiple Choice Questions (MCQs)
1. Pioglitazone primarily works by activating which receptor?
a) GLP-1 receptor
b) Insulin receptor
c) PPAR-γ receptor
d) AMPK
Answer: c) PPAR-γ receptor
2. Which of the following is a side effect of pioglitazone?
a) Hypoglycemia
b) Edema
c) Hyperkalemia
d) Pancreatitis
Answer: b) Edema
3. Pioglitazone enhances glucose uptake primarily in:
a) Pancreas
b) Kidney
c) Liver
d) Muscle and adipose tissue
Answer: d) Muscle and adipose tissue
4. Which gene expression does pioglitazone affect to increase glucose transport?
a) GLUT2
b) GLUT4
c) SGLT1
d) Insulin
Answer: b) GLUT4
5. Pioglitazone is contraindicated in which condition?
a) Type 1 diabetes
b) Obesity
c) Congestive heart failure (Class III/IV)
d) Hypertension
Answer: c) Congestive heart failure (Class III/IV)
FAQs
Q1. Can pioglitazone be combined with insulin?
Yes, but the risk of fluid retention and heart failure increases.
Q2. Is pioglitazone effective in type 1 diabetes?
No, it requires functioning pancreatic β-cells to be effective.
Q3. How does pioglitazone affect lipids?
It increases HDL and reduces triglycerides.
Q4. What is the typical dosing schedule?
Usually once daily, with or without food.
Q5. Does pioglitazone improve insulin resistance?
Yes, it is specifically indicated to reduce insulin resistance in peripheral tissues.
References
- Goodman & Gilman’s The Pharmacological Basis of Therapeutics, 12th Edition
- KD Tripathi, Essentials of Medical Pharmacology, 7th Edition
- Prescribing information for Pioglitazone
- American Diabetes Association Guidelines
Related Internal Links

I am pursuing MBA in pharmaceutical management from NIPER Hyderabad with a strong academic record and proven success in national-level pharmacy entrance exams. I secured AIR 61 in NIPER 2024 (MS/M.Pharm) and AIR 27 in NIPER MBA, along with AIR 147 in GPAT 2024 and AIR 907 in GPAT 2023. I also achieved AIR 6 in AIIMS CRE-2025 for Drug Store Keeper and was selected as a Pharmacist (AIR 61) for ESIC. Additionally, I was the Runner-Up in Round 2 of the EY Case Study Competition.
At PharmacyFreak.com, I aim to guide future pharmacists through expert content, exam strategies, and insightful resources based on real experience and academic excellence.
Mail- harsh@pharmacyfreak.com