Table of Contents
Introduction
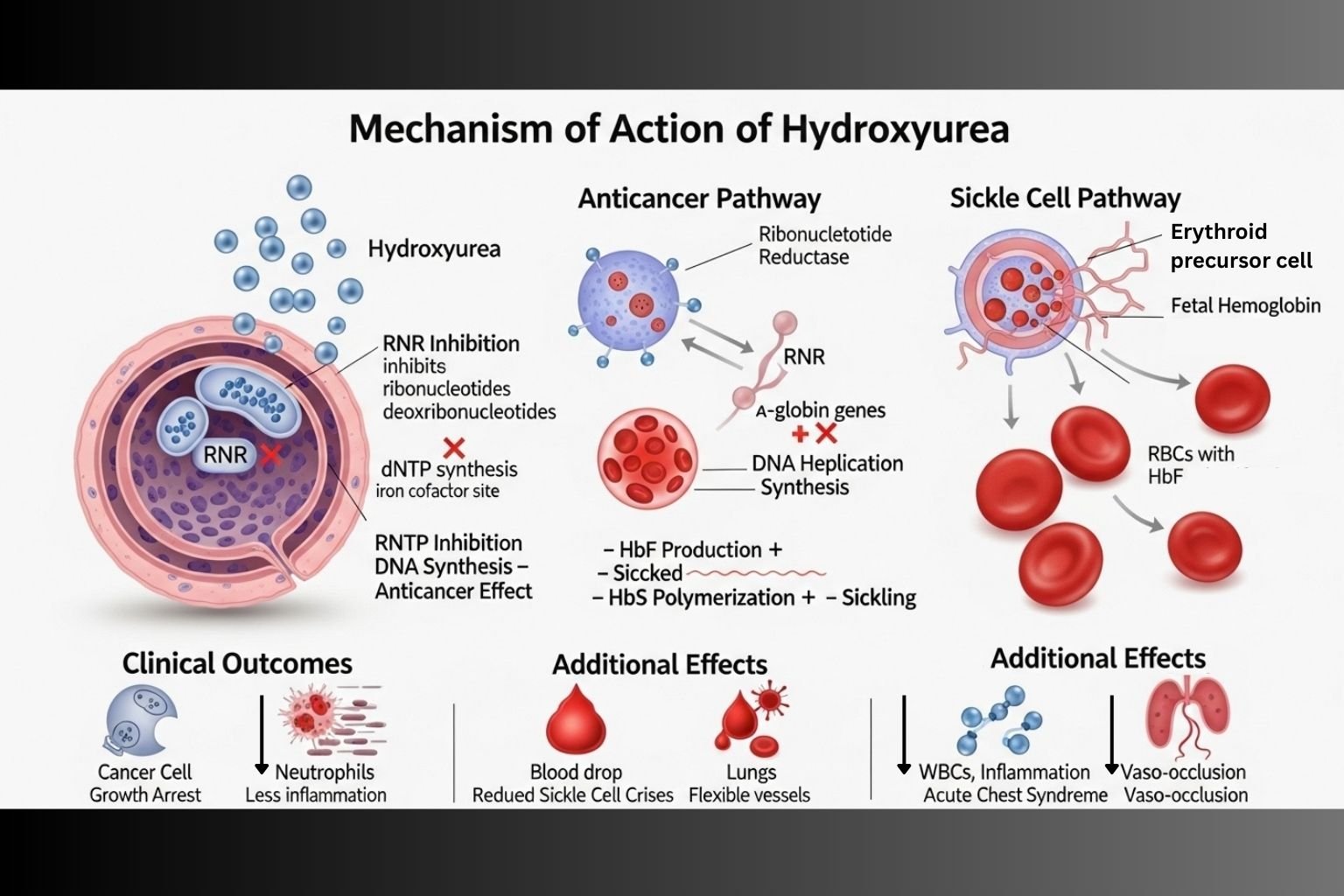
Hydroxyurea is an antineoplastic and hematologic agent used in the treatment of several conditions including chronic myelogenous leukemia (CML), sickle cell anemia, and certain solid tumors. Its therapeutic effectiveness is derived from its ability to inhibit DNA synthesis and modulate erythropoiesis. Hydroxyurea’s distinct dual mechanism—cytotoxicity in cancer and fetal hemoglobin induction in sickle cell disease—makes it a versatile agent.
Mechanism of Action (Step-wise)
1. Inhibition of Ribonucleotide Reductase
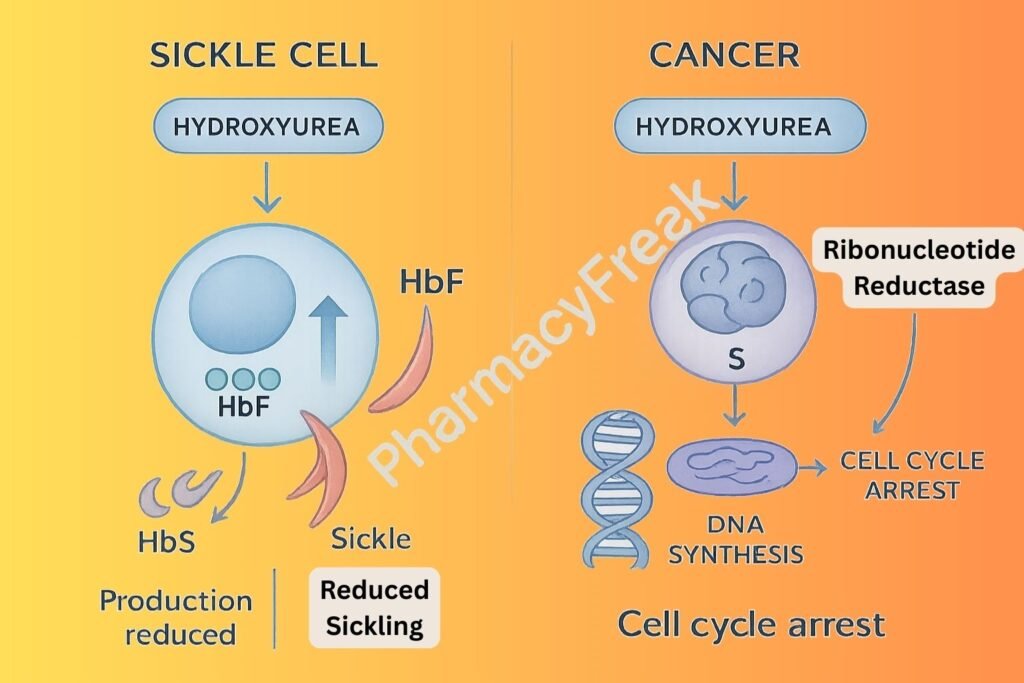
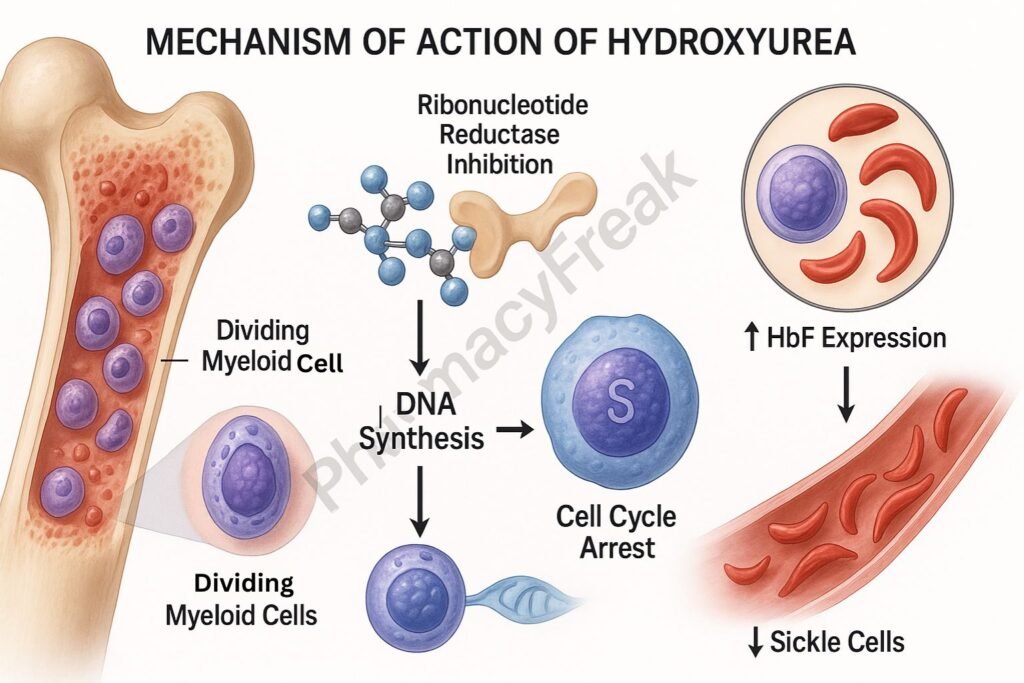
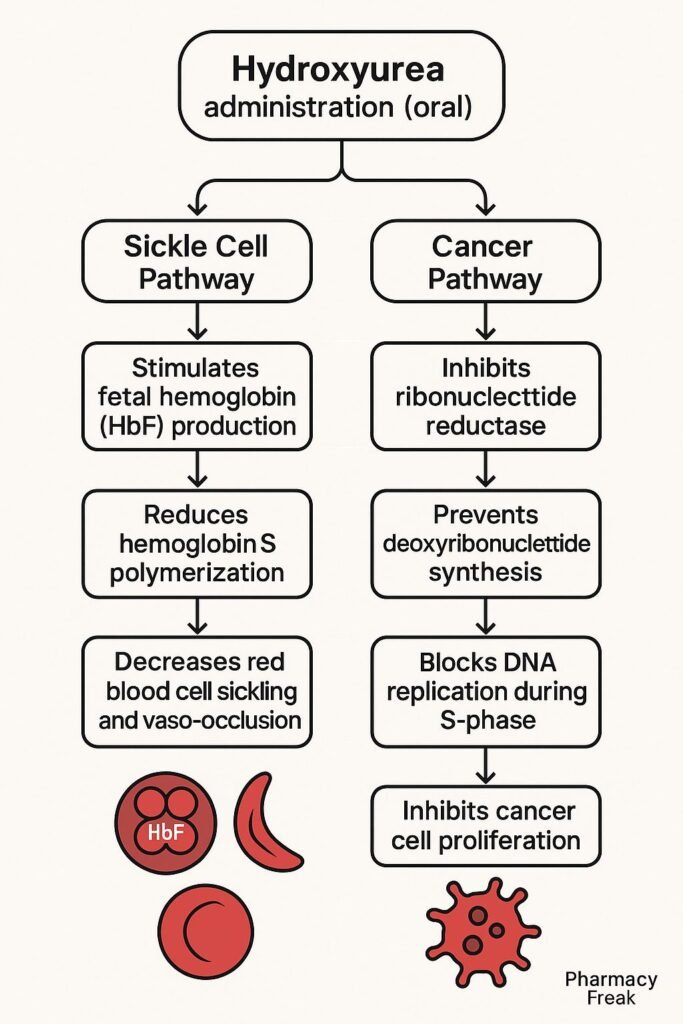
Hydroxyurea selectively inhibits ribonucleotide reductase, an enzyme essential for converting ribonucleotides to deoxyribonucleotides.
2. Disruption of DNA Synthesis
By reducing deoxynucleotide pools, hydroxyurea inhibits DNA replication, leading to cell cycle arrest in the S-phase.
3. Cytotoxic Effects on Rapidly Dividing Cells
Cells that are rapidly dividing, such as in leukemia or certain tumors, are especially sensitive to hydroxyurea-induced cytotoxicity.
4. Induction of Fetal Hemoglobin (HbF) Production
In sickle cell disease, hydroxyurea stimulates the production of HbF, which interferes with the polymerization of sickle hemoglobin (HbS), thereby reducing sickling.
5. Reduction in Leukocyte and Platelet Counts
Hydroxyurea causes mild bone marrow suppression, beneficial in hyperproliferative disorders.
Pharmacokinetics
- Route of Administration: Oral
- Bioavailability: ~80–100%
- Time to Peak Plasma: 1–4 hours
- Half-Life: 3–9 hours
- Metabolism: Hepatic (minor); primarily excreted unchanged
- Excretion: Renal (mainly unchanged drug)
Clinical Uses
- Sickle cell anemia (to reduce frequency of pain crises)
- Chronic myelogenous leukemia (CML)
- Essential thrombocythemia and polycythemia vera
- Head and neck cancers (in combination therapy)
- Psoriasis (off-label)
Adverse Effects
- Myelosuppression (leukopenia, anemia, thrombocytopenia)
- Gastrointestinal upset
- Skin hyperpigmentation and nail changes
- Leg ulcers (long-term use)
- Teratogenicity and infertility
- Contraindications: Pregnancy, severe bone marrow suppression, hypersensitivity
Comparative Analysis
| Parameter | Hydroxyurea | Imatinib |
|---|---|---|
| Primary Use | CML, Sickle cell disease | CML |
| Mechanism | Ribonucleotide reductase inhibitor | Tyrosine kinase inhibitor |
| DNA Synthesis | Inhibited | Not directly affected |
| Fetal Hemoglobin Effect | Yes | No |
| Myelosuppression | Yes | Less common |
Multiple Choice Questions (MCQs)
1. Hydroxyurea inhibits which enzyme?
a) DNA polymerase
b) Ribonucleotide reductase
c) Thymidylate synthase
d) Topoisomerase II
Answer: b) Ribonucleotide reductase
2. The clinical benefit of hydroxyurea in sickle cell disease is due to:
a) Bone marrow suppression
b) Fetal hemoglobin induction
c) Iron chelation
d) Anti-inflammatory action
Answer: b) Fetal hemoglobin induction
3. Hydroxyurea arrests the cell cycle at which phase?
a) G0
b) G1
c) S-phase
d) M-phase
Answer: c) S-phase
4. A long-term dermatologic side effect of hydroxyurea is:
a) Alopecia
b) Psoriasis
c) Leg ulcers
d) Urticaria
Answer: c) Leg ulcers
5. Which condition is NOT an approved use of hydroxyurea?
a) Sickle cell anemia
b) CML
c) Psoriasis
d) Systemic lupus erythematosus
Answer: d) Systemic lupus erythematosus
FAQs
Q1. How long does hydroxyurea take to show effects in sickle cell anemia?
Usually within 3–6 months of consistent use.
Q2. Is hydroxyurea safe in pregnancy?
No, it is contraindicated due to teratogenic effects.
Q3. How is myelosuppression monitored during therapy?
Regular complete blood counts (CBCs) are essential.
Q4. Can hydroxyurea prevent stroke in sickle cell patients?
Yes, it reduces the risk of stroke in high-risk pediatric populations.
Q5. What is the usual dosing frequency?
Typically once daily, adjusted based on patient response and toxicity.
References
- Goodman & Gilman’s The Pharmacological Basis of Therapeutics, 12th Edition
- KD Tripathi, Essentials of Medical Pharmacology, 7th Edition
- Hydroxyurea Prescribing Information
- Clinical guidelines for sickle cell anemia and CML
Related Internal Links

I am pursuing MBA in pharmaceutical management from NIPER Hyderabad with a strong academic record and proven success in national-level pharmacy entrance exams. I secured AIR 61 in NIPER 2024 (MS/M.Pharm) and AIR 27 in NIPER MBA, along with AIR 147 in GPAT 2024 and AIR 907 in GPAT 2023. I also achieved AIR 6 in AIIMS CRE-2025 for Drug Store Keeper and was selected as a Pharmacist (AIR 61) for ESIC. Additionally, I was the Runner-Up in Round 2 of the EY Case Study Competition.
At PharmacyFreak.com, I aim to guide future pharmacists through expert content, exam strategies, and insightful resources based on real experience and academic excellence.
Mail- harsh@pharmacyfreak.com