Table of Contents
Introduction
Fluticasone is a synthetic corticosteroid belonging to the glucocorticoid class, widely used for its potent anti-inflammatory and immunosuppressive effects. It is commonly administered via inhalation, intranasal spray, or topical routes for the management of asthma, allergic rhinitis, and other inflammatory conditions. Fluticasone is high-yield for pharmacology and clinical exams because of its receptor-mediated genomic actions, high topical potency, and minimal systemic effects when used correctly.
Mechanism of Action (Step-wise)
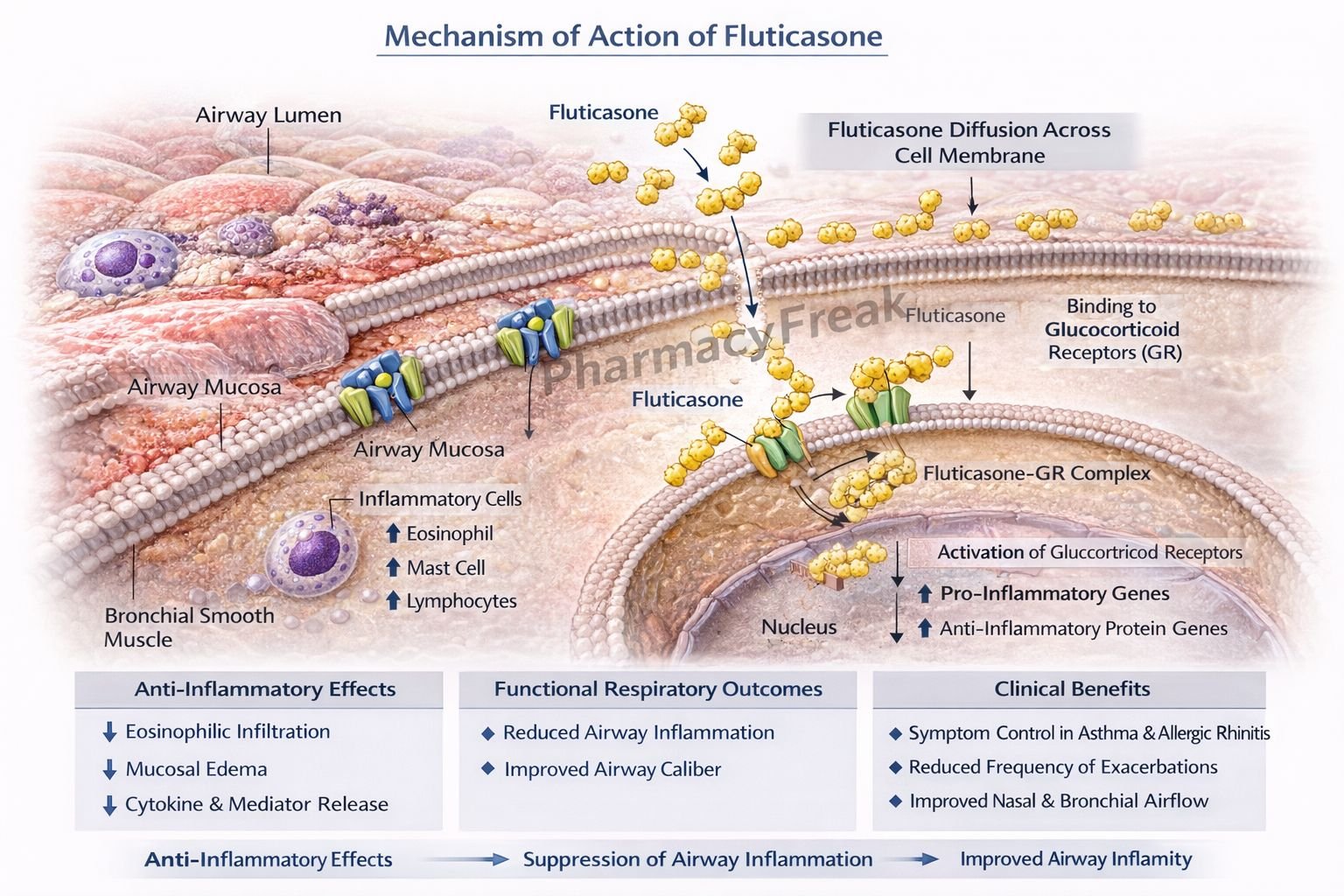
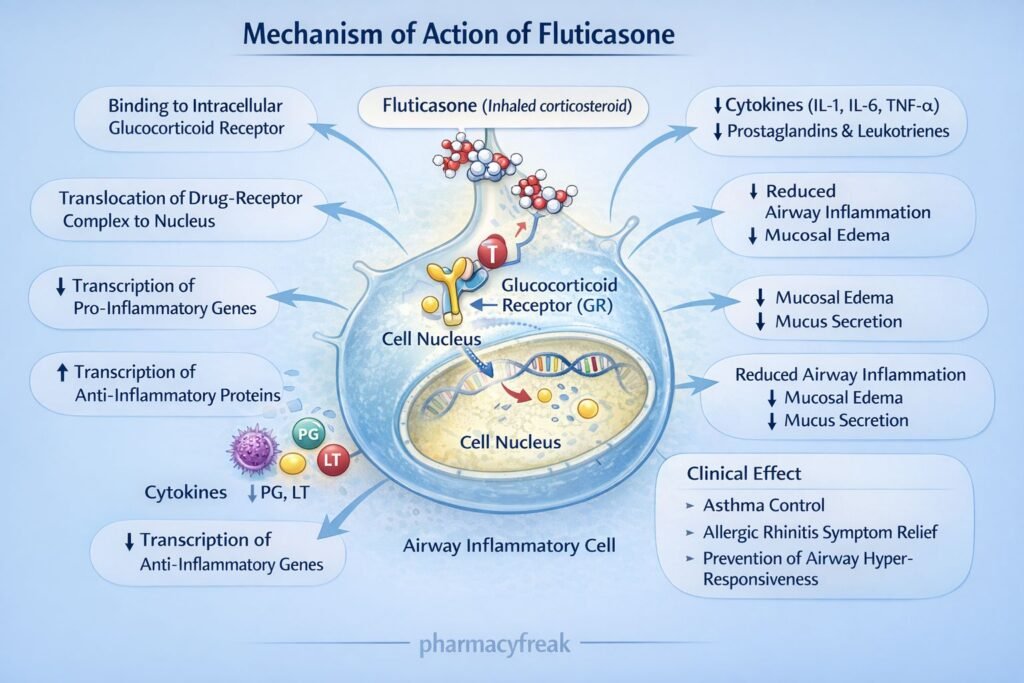
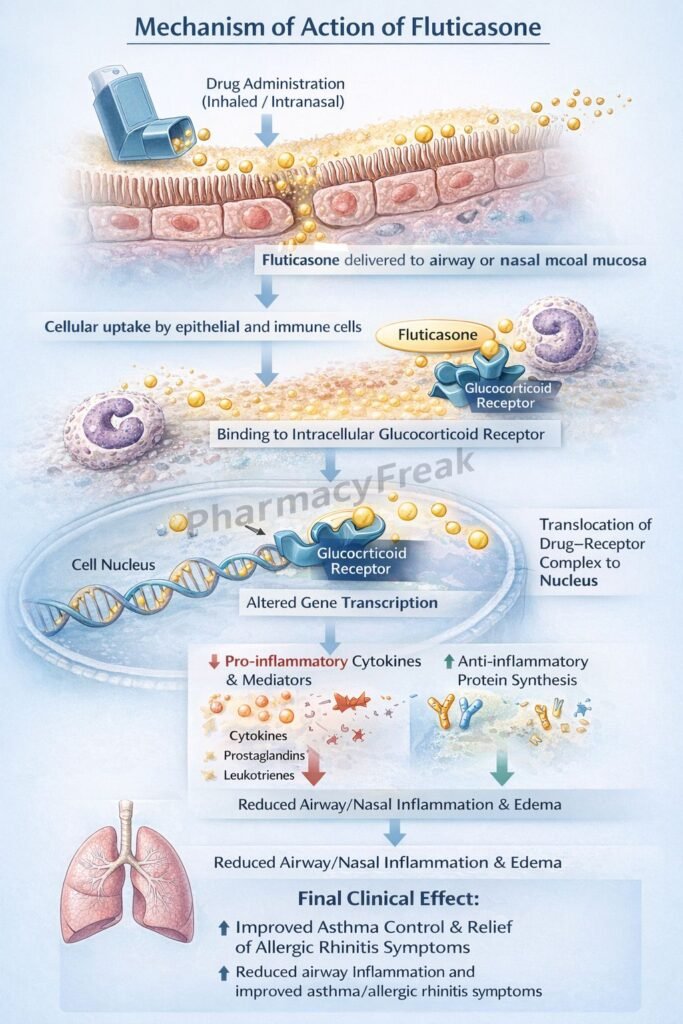
Fluticasone exerts anti-inflammatory effects by modulating gene transcription via glucocorticoid receptors.
Step 1: Entry into target cells
Fluticasone diffuses across cell membranes due to its lipophilic nature.
Step 2: Binding to glucocorticoid receptors (GR)
It binds to cytoplasmic glucocorticoid receptors, forming a drug–receptor complex.
Step 3: Nuclear translocation
The fluticasone–GR complex translocates into the nucleus.
Step 4: Regulation of gene transcription
- Upregulates anti-inflammatory genes (e.g., lipocortin-1)
- Downregulates pro-inflammatory genes (e.g., cytokines, chemokines, COX-2)
Step 5: Inhibition of inflammatory mediators
Lipocortin-1 inhibits phospholipase A₂, reducing synthesis of prostaglandins and leukotrienes.
Step 6: Reduced airway inflammation and hyperresponsiveness
Decreased edema, mucus secretion, and inflammatory cell infiltration result in improved airway function.
Exam pearl:
Fluticasone has no direct bronchodilator action.
Pharmacokinetics
- Route of administration: Inhaled, intranasal, topical
- Oral bioavailability: <1% (extensive first-pass metabolism)
- Protein binding: ~90%
- Distribution: High local tissue concentration
- Metabolism: Hepatic (CYP3A4)
- Half-life: ~7–8 hours
- Excretion: Feces (major), urine (minor)
Clinical Uses
- Bronchial asthma (maintenance therapy)
- Allergic rhinitis (intranasal spray)
- Chronic obstructive pulmonary disease (COPD) (combination inhalers)
- Atopic dermatitis (topical formulation)
- Nasal polyps
Fluticasone is used for prophylaxis and control, not for acute asthma attacks.
Adverse Effects
Local (most common):
- Oral candidiasis
- Dysphonia
- Throat irritation
- Nasal dryness or epistaxis (intranasal)
Systemic (high dose/prolonged use):
- Adrenal suppression
- Growth retardation in children
- Osteoporosis (rare)
Exam warning:
Rinse mouth after inhalation to prevent oral thrush.
Comparative Analysis
Fluticasone vs Budesonide vs Prednisolone
| Feature | Fluticasone | Budesonide | Prednisolone |
|---|---|---|---|
| Route | Inhaled/topical | Inhaled/oral | Oral/IV |
| First-pass metabolism | Extensive | Extensive | Minimal |
| Systemic effects | Very low | Low | High |
| Use in asthma | Maintenance | Maintenance | Acute/severe |
| Anti-inflammatory potency | Very high | High | Moderate |
Explanation:
Fluticasone’s high topical potency and extensive first-pass metabolism make it ideal for chronic inflammatory airway diseases with minimal systemic toxicity, unlike systemic corticosteroids such as prednisolone.
MCQs
- Fluticasone primarily binds to:
a) Mineralocorticoid receptors
b) Estrogen receptors
c) Glucocorticoid receptors
d) β₂ receptors
Answer: c) Glucocorticoid receptors
- Anti-inflammatory action of fluticasone involves inhibition of:
a) Cyclooxygenase directly
b) Phospholipase A₂
c) Lipoxygenase
d) Histamine release
Answer: b) Phospholipase A₂
- Fluticasone is mainly used in asthma as:
a) Rescue therapy
b) Bronchodilator
c) Maintenance therapy
d) Emergency drug
Answer: c) Maintenance therapy
- Which adverse effect is most common with inhaled fluticasone?
a) Hypertension
b) Oral candidiasis
c) Hyperglycemia
d) Cataract
Answer: b) Oral candidiasis
- Fluticasone has minimal systemic effects because of:
a) Poor receptor binding
b) Renal excretion
c) Extensive first-pass metabolism
d) Low potency
Answer: c) Extensive first-pass metabolism
- Fluticasone reduces leukotriene synthesis by:
a) Blocking COX
b) Blocking LOX
c) Inhibiting phospholipase A₂
d) Blocking histamine
Answer: c) Inhibiting phospholipase A₂
- Fluticasone has no effect on:
a) Cytokine production
b) Airway inflammation
c) Bronchial smooth muscle tone
d) Eosinophil migration
Answer: c) Bronchial smooth muscle tone
- CYP enzyme involved in fluticasone metabolism is:
a) CYP2D6
b) CYP2C19
c) CYP3A4
d) CYP1A2
Answer: c) CYP3A4
- Fluticasone is preferred in asthma because it:
a) Acts rapidly
b) Causes bronchodilation
c) Reduces airway inflammation
d) Stimulates β₂ receptors
Answer: c) Reduces airway inflammation
- Growth suppression risk with fluticasone is:
a) Very high
b) Common
c) Dose-dependent and minimal
d) Absent
Answer: c) Dose-dependent and minimal
FAQs
1. Does fluticasone relieve acute asthma attacks?
No, it is for long-term control only.
2. Why should mouth rinsing be advised?
To prevent oral candidiasis.
3. Can fluticasone suppress adrenal function?
Yes, at high doses or prolonged use.
4. Is fluticasone safe in children?
Yes, with appropriate dosing and monitoring.
5. Why is fluticasone preferred over oral steroids?
Due to minimal systemic side effects.
References
- Goodman & Gilman’s The Pharmacological Basis of Therapeutics
https://accesspharmacy.mhmedical.com - Katzung BG. Basic and Clinical Pharmacology
https://accessmedicine.mhmedical.com - Tripathi KD. Essentials of Medical Pharmacology
- Harrison’s Principles of Internal Medicine
https://accessmedicine.mhmedical.com

I am pursuing MBA in pharmaceutical management from NIPER Hyderabad with a strong academic record and proven success in national-level pharmacy entrance exams. I secured AIR 61 in NIPER 2024 (MS/M.Pharm) and AIR 27 in NIPER MBA, along with AIR 147 in GPAT 2024 and AIR 907 in GPAT 2023. I also achieved AIR 6 in AIIMS CRE-2025 for Drug Store Keeper and was selected as a Pharmacist (AIR 61) for ESIC. Additionally, I was the Runner-Up in Round 2 of the EY Case Study Competition.
At PharmacyFreak.com, I aim to guide future pharmacists through expert content, exam strategies, and insightful resources based on real experience and academic excellence.
Mail- harsh@pharmacyfreak.com