Table of Contents
Introduction
Cyclophosphamide is a nitrogen mustard–derived alkylating agent widely used in oncology and immunosuppressive therapy. It is classified as a cell cycle–nonspecific cytotoxic drug and is used in the treatment of a broad spectrum of malignancies, as well as autoimmune and inflammatory disorders. Cyclophosphamide is a prodrug that requires hepatic activation, and its DNA-alkylating mechanism makes it a high-yield topic for pharmacology, oncology, hematology, and clinical entrance examinations.
Mechanism of Action (Step-wise)
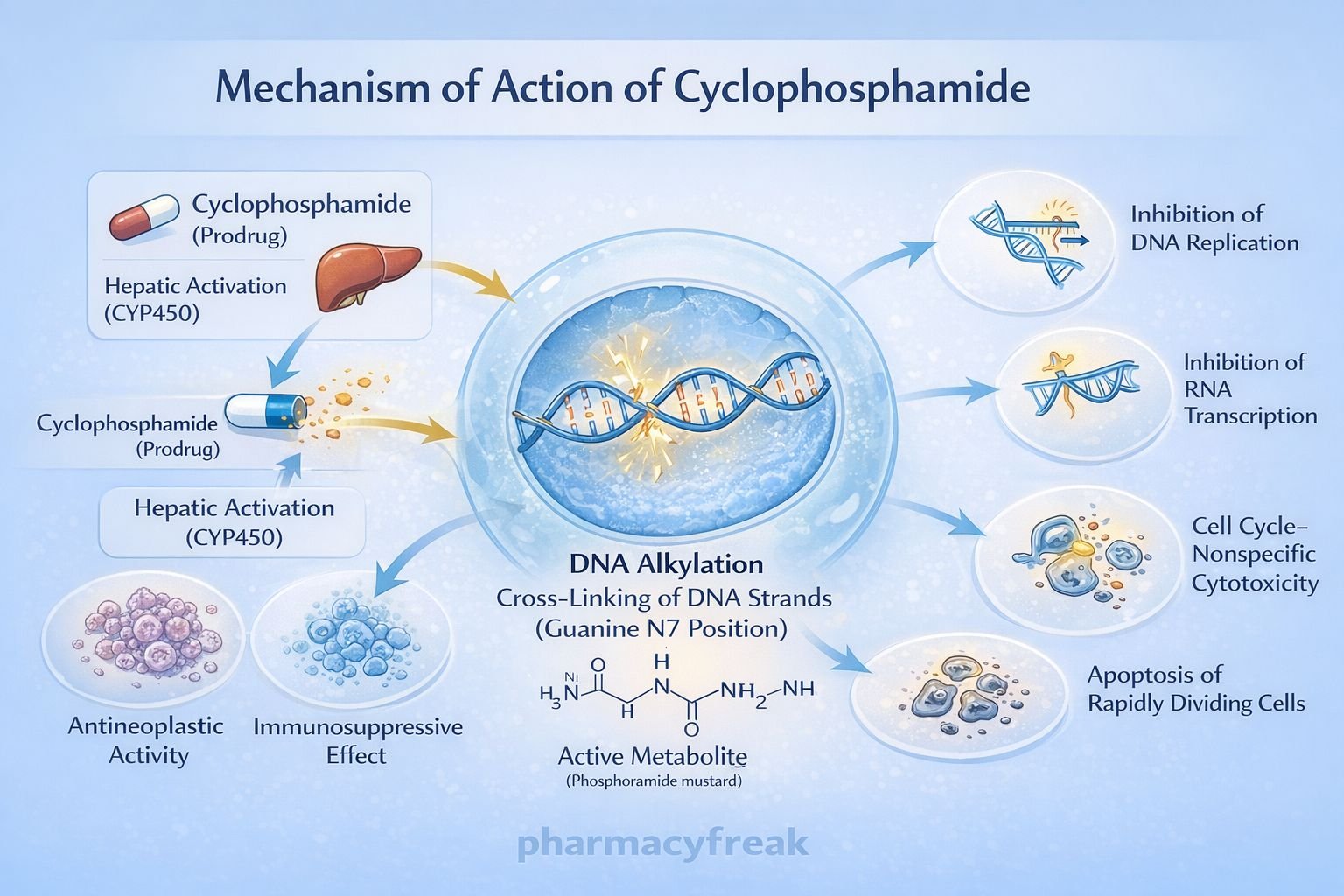
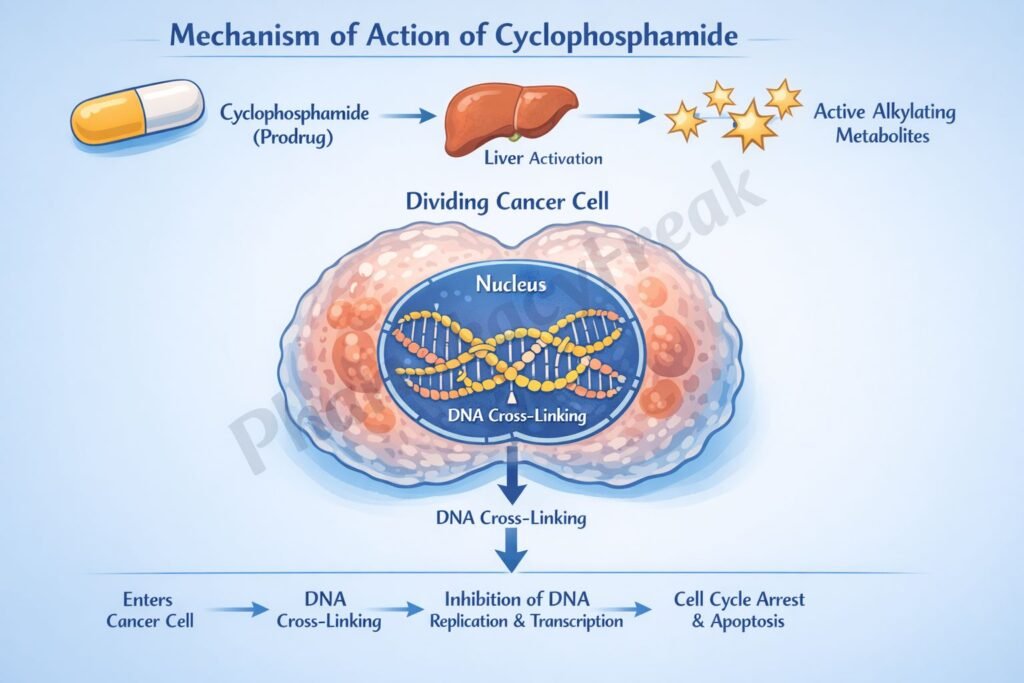
Cyclophosphamide exerts cytotoxic and immunosuppressive effects through DNA alkylation after metabolic activation.
Step-wise mechanism:
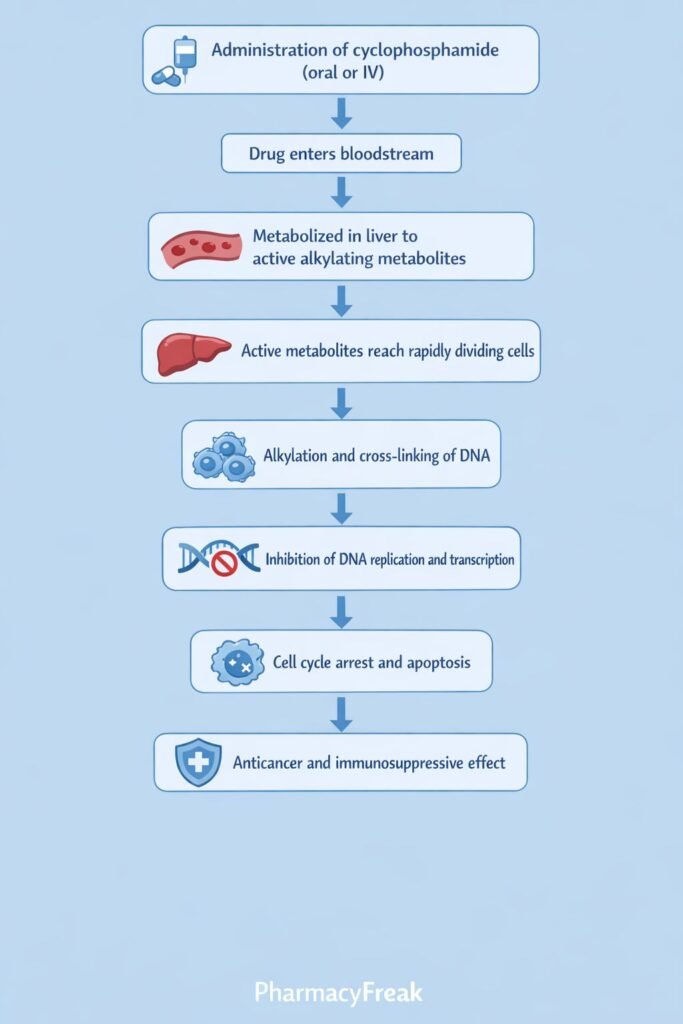
- Prodrug Administration
Cyclophosphamide is administered orally or intravenously in an inactive form. - Hepatic Activation
In the liver, cyclophosphamide is metabolized by cytochrome P450 enzymes (mainly CYP2B6, CYP3A4) to active metabolites:- 4-hydroxycyclophosphamide
- Aldophosphamide
- Formation of Active Cytotoxic Metabolites
Aldophosphamide is further converted into:- Phosphoramide mustard (active antineoplastic agent)
- Acrolein (toxic byproduct)
- DNA Alkylation
Phosphoramide mustard forms covalent bonds with the N7 position of guanine bases in DNA. - DNA Cross-Linking
Interstrand and intrastrand DNA cross-links are formed, preventing DNA strand separation. - Inhibition of DNA Replication and Transcription
Cross-linked DNA cannot replicate or transcribe effectively. - Cell Death
Rapidly dividing cells undergo apoptosis due to irreparable DNA damage. - Immunosuppressive Effect
Preferential destruction of proliferating B and T lymphocytes leads to suppression of humoral and cellular immunity.
Pharmacokinetics
- Absorption: Well absorbed orally
- Distribution: Widely distributed; crosses blood–brain barrier and placenta
- Metabolism: Hepatic activation via CYP450 enzymes
- Elimination: Renal excretion of metabolites
- Half-life: Approximately 7 hours
- Special note: Acrolein accumulates in urine and causes bladder toxicity
Adequate hydration and coadministration of mesna reduce urotoxicity.
Clinical Uses
Cyclophosphamide is used in both malignant and non-malignant conditions:
Oncologic uses
- Lymphomas (Hodgkin and non-Hodgkin)
- Leukemias
- Breast cancer
- Ovarian cancer
- Multiple myeloma
Non-oncologic uses
- Systemic lupus erythematosus
- Vasculitis (e.g., granulomatosis with polyangiitis)
- Nephrotic syndrome
- Prevention of transplant rejection
Adverse Effects
Cyclophosphamide has dose-limiting and characteristic toxicities:
- Hematologic:
- Myelosuppression
- Leukopenia
- Urotoxicity:
- Hemorrhagic cystitis (due to acrolein)
- Gastrointestinal:
- Nausea
- Vomiting
- Reproductive:
- Gonadal suppression
- Infertility
- Others:
- Alopecia
- Secondary malignancies (long-term use)
Comparative Analysis (must include a table + explanation)
Comparison of Alkylating Agents
| Feature | Cyclophosphamide | Cisplatin | Chlorambucil |
|---|---|---|---|
| Prodrug | Yes | No | No |
| Activation | Hepatic CYP | Non-enzymatic | Direct |
| DNA cross-linking | Yes | Yes | Yes |
| Major toxicity | Hemorrhagic cystitis | Nephrotoxicity | Myelosuppression |
| Immunosuppressive use | Yes | No | Limited |
Explanation:
Cyclophosphamide differs from other alkylating agents by requiring hepatic activation and having prominent immunosuppressive effects. Its unique urotoxicity due to acrolein distinguishes it clinically, while cisplatin is limited by nephrotoxicity and chlorambucil by bone marrow suppression.
MCQs (10–15)
- Cyclophosphamide is classified as a:
a) Antimetabolite
b) Alkylating agent
c) Mitotic inhibitor
d) Topoisomerase inhibitor
Answer: b) Alkylating agent
- Cyclophosphamide requires activation in the:
a) Kidney
b) Bone marrow
c) Liver
d) Tumor cell
Answer: c) Liver
- The active cytotoxic metabolite of cyclophosphamide is:
a) Acrolein
b) Phosphoramide mustard
c) Aldophosphamide
d) Nitrosourea
Answer: b) Phosphoramide mustard
- Cyclophosphamide kills cells by:
a) Inhibiting mitosis
b) Blocking folate metabolism
c) Cross-linking DNA
d) Inhibiting RNA polymerase
Answer: c) Cross-linking DNA
- Hemorrhagic cystitis is caused by:
a) Phosphoramide mustard
b) Mesna
c) Acrolein
d) Urea
Answer: c) Acrolein
- Which drug is used to prevent cyclophosphamide-induced cystitis?
a) Folic acid
b) Leucovorin
c) Mesna
d) Allopurinol
Answer: c) Mesna
- Cyclophosphamide is cell cycle–specific:
a) Only in S phase
b) Only in M phase
c) Only in G1 phase
d) No
Answer: d) No
- Cyclophosphamide is commonly used in:
a) Solid tumors only
b) Autoimmune diseases
c) Viral infections
d) Parkinson disease
Answer: b) Autoimmune diseases
- Which system is most affected by cyclophosphamide toxicity?
a) Nervous system
b) Cardiovascular system
c) Bone marrow
d) Endocrine system
Answer: c) Bone marrow
- Long-term cyclophosphamide therapy increases the risk of:
a) Hypertension
b) Diabetes
c) Secondary malignancies
d) Hyperthyroidism
Answer: c) Secondary malignancies
FAQs (minimum 5)
- Is cyclophosphamide a prodrug?
Yes, it requires hepatic activation to become cytotoxic. - Why is mesna coadministered with cyclophosphamide?
To prevent hemorrhagic cystitis caused by acrolein. - Does cyclophosphamide affect the immune system?
Yes, it suppresses both B and T lymphocytes. - Is cyclophosphamide cell cycle–specific?
No, it is cell cycle–nonspecific. - Can cyclophosphamide cause infertility?
Yes, due to gonadal toxicity. - Why is hydration important during therapy?
To reduce bladder toxicity from acrolein.
References
- Goodman & Gilman’s The Pharmacological Basis of Therapeutics
https://accessmedicine.mhmedical.com - Katzung BG. Basic and Clinical Pharmacology
https://accessmedicine.mhmedical.com - Tripathi KD. Essentials of Medical Pharmacology
https://www.jaypeebrothers.com - Harrison’s Principles of Internal Medicine
https://accessmedicine.mhmedical.com

I am pursuing MBA in pharmaceutical management from NIPER Hyderabad with a strong academic record and proven success in national-level pharmacy entrance exams. I secured AIR 61 in NIPER 2024 (MS/M.Pharm) and AIR 27 in NIPER MBA, along with AIR 147 in GPAT 2024 and AIR 907 in GPAT 2023. I also achieved AIR 6 in AIIMS CRE-2025 for Drug Store Keeper and was selected as a Pharmacist (AIR 61) for ESIC. Additionally, I was the Runner-Up in Round 2 of the EY Case Study Competition.
At PharmacyFreak.com, I aim to guide future pharmacists through expert content, exam strategies, and insightful resources based on real experience and academic excellence.
Mail- harsh@pharmacyfreak.com