Table of Contents
1. Identification
Summary
Ibuprofen is a widely used nonsteroidal anti-inflammatory drug (NSAID) indicated for relief of mild–moderate pain, fever, and inflammatory conditions; available OTC in many regions and by prescription for higher strengths/formulations.
Brand Names
Advil, Motrin, Nurofen, Brufen (regional variants and numerous generics)
Name
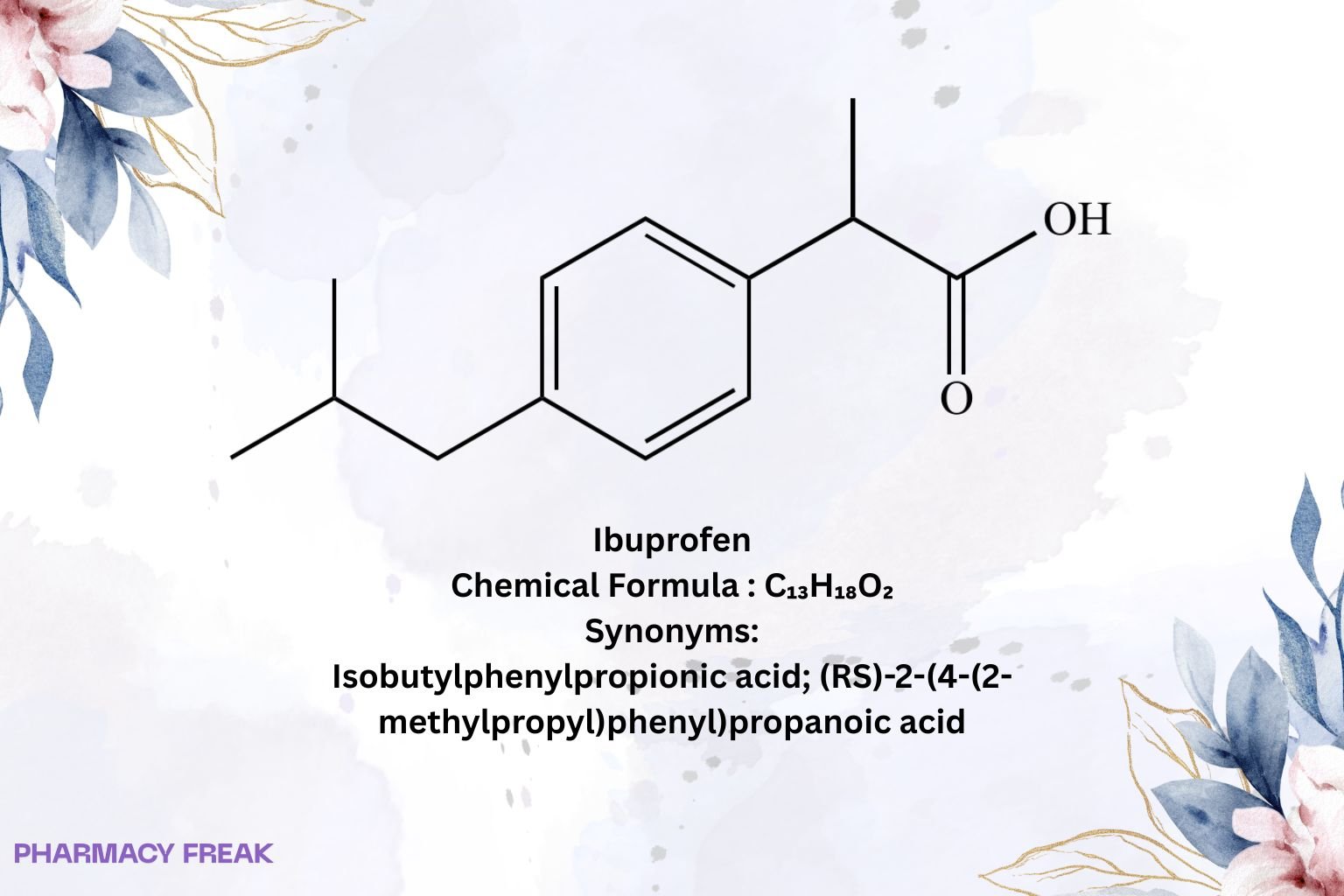
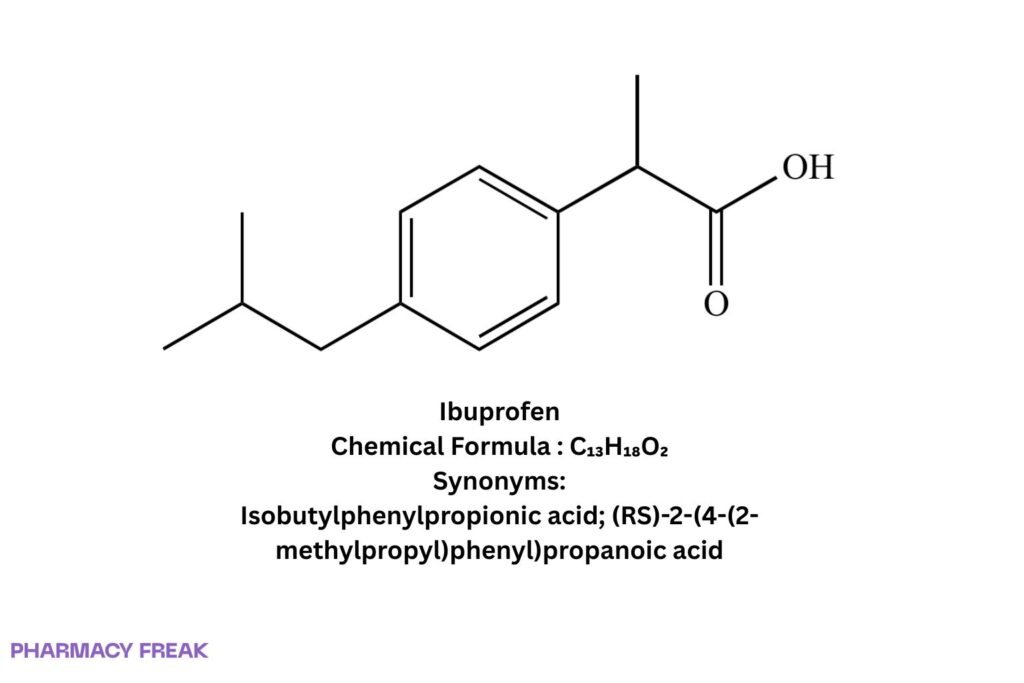
Ibuprofen
Background
A propionic-acid NSAID introduced in the late 1960s/1970s; supplied as a racemic mixture. Oral tablets/capsules, suspensions, topical products, and IV (certain jurisdictions) are available.
Modality
Small molecule
Groups
Approved; OTC/Rx (region-dependent)
Structure
Weight
~206.28 g/mol
Chemical Formula
C₁₃H₁₈O₂
Synonyms
Isobutylphenylpropionic acid; (RS)-2-(4-(2-methylpropyl)phenyl)propanoic acid
External IDs
CAS: 15687-27-1; PubChem CID: 3672; UNII: WK2XYI10QM; KEGG: D00126; ChEMBL: 521; ChEBI: 5855
2. Pharmacology
Indication
Short-term management of pain (e.g., headache, dental, musculoskeletal, dysmenorrhoea), fever, and inflammatory conditions (e.g., osteoarthritis/RA symptoms). Certain formulations are used in hospitals; some IV/lysine forms are approved for specific uses per local labeling.
Associated Conditions
Fever, primary dysmenorrhoea, musculoskeletal/soft-tissue pain, postoperative pain, migraine, osteoarthritis flares.
Associated Therapies
Used within multimodal analgesia with acetaminophen/paracetamol or adjuvants; various fixed-dose combinations exist regionally.
Contraindications & Blackbox Warnings
Class boxed warnings for NSAIDs: ↑ risk of serious cardiovascular thrombotic events (MI, stroke) and GI bleeding/ulceration/perforation; contraindicated for peri-operative pain in CABG setting. Avoid with history of NSAID/aspirin hypersensitivity, active GI bleeding, and in certain renal risks per label.
Pharmacodynamics
Reversible, non-selective inhibition of COX-1/COX-2, reducing prostaglandin synthesis → analgesic, antipyretic, anti-inflammatory effects.
Mechanism of action
Competitive COX blockade decreases prostaglandins and thromboxane formation; antipyresis via central action; analgesia via peripheral/central prostaglandin reduction.
Absorption
Rapid after oral dosing; food may delay tₘₐₓ and reduce Cmax, but total exposure typically similar for IR products; taking with food improves GI tolerability.
Volume of distribution
Relatively low (on the order of ~0.1–0.2 L/kg), consistent with strong protein binding and limited tissue partitioning.
Protein binding
High—~98–99% at therapeutic concentrations.
Metabolism
Hepatic, mainly CYP2C9 to hydroxylated and carboxylated metabolites; conjugation (glucuronides) follows.
Route of elimination
Predominantly renal, largely as metabolites (a small fraction unchanged).
Half-life
Typically ~2–4 hours (formulation/individual factors may vary).
Clearance
Primarily renal for metabolites; apparent oral clearance is influenced by protein binding, hepatic biotransformation, and renal function.
Adverse Effects
Common: dyspepsia, heartburn, nausea, abdominal pain, dizziness, rash. Serious: GI bleeding/ulcer, CV thrombotic events, renal events, anaphylaxis/bronchospasm in aspirin-sensitive individuals, severe cutaneous reactions (rare).
Toxicity
Overdose may cause GI symptoms, CNS depression, metabolic acidosis, renal compromise; management is supportive with attention to airway/hemodynamics and complications.
Pathways
COX-1/COX-2 inhibition; downstream reduction of PGE₂/PGI₂/TXA₂; renal prostaglandin modulation relevant to fluid/electrolyte balance and renal blood flow.
Pharmacogenomic Effects/ADRs
CYP2C9 polymorphisms can affect metabolism/exposure; aspirin-exacerbated respiratory disease (AERD) reflects clinical susceptibility rather than a single gene determinant.
3. Interactions
Drug Interactions
- Aspirin (low-dose): Ibuprofen may interfere with aspirin’s antiplatelet effect—timing separation is often advised in labels/agency guidance.
- Anticoagulants/antiplatelets (e.g., warfarin): ↑ bleeding risk; monitor.
- ACEIs/ARBs/diuretics (“triple whammy”): additive renal risk; monitor renal function.
- Lithium / Methotrexate: potential ↑ levels/toxicity; monitor.
- SSRIs/SNRIs, corticosteroids: additive GI bleeding risk.
Food Interactions
May be taken with food to reduce GI upset; food can delay peak absorption but usually doesn’t meaningfully reduce overall exposure for IR products. Alcohol co-use increases GI risk.
4. Categories
ATC Codes
M01AE01 (ibuprofen; anti-inflammatory and antirheumatic products, non-steroids)
Drug Categories
NSAID; Analgesic; Antipyretic; Anti-inflammatory; Small molecule
Chemical Taxonomy
Propionic-acid derivative; aromatic carboxylic acid; racemate (R/S)
Affected organisms
Humans (therapeutic use)
5. Chemical Identifiers
UNII
WK2XYI10QM
CAS number
15687-27-1
InChI Key
HEFNNWSXXWATRW-UHFFFAOYSA-N
InChI
InChI=1S/C13H18O2/c1-9(2)8-11-4-6-12(7-5-11)10(3)13(14)15/h4-7,9-10H,8H2,1-3H3,(H,14,15)
IUPAC Name
(RS)-2-(4-(2-methylpropyl)phenyl)propanoic acid
SMILES
CC(C)Cc1ccc(cc1)C(C)C(=O)O
6. References
- PubChem Compound Summary: Ibuprofen (CID 3672) — formula, molecular weight, identifiers. PubChem
- FDA GSRS / precision.fda: UNII WK2XYI10QM (Ibuprofen) — UNII, InChIKey, formula. precision.fda.gov+1
- StatPearls: Ibuprofen — PK (food effect, tₘₐₓ), distribution, protein binding (~99%), clinical use overview. NCBI
- DailyMed (multiple ibuprofen labels) — NSAID boxed warnings (CV/GI), CABG contraindication, consumer/HC-professional labeling. DailyMed+2DailyMed+2
- FDA Safety Communication / Q&A: Ibuprofen with low-dose aspirin — potential antiplatelet interference and timing considerations. U.S. Food and Drug Administration+1
- NIST WebBook: Ibuprofen — IUPAC Standard InChI and InChIKey. NIST WebBook
- Sigma-Aldrich (product page) — canonical SMILES, InChI string confirmation. MilliporeSigma
- ATC/DDD Index: M01AE01 (ibuprofen) — ATC classification, DDD/routes. atcddd.fhi.no
- WHO eEML (Electronic Essential Medicines List): Ibuprofen — essential-medicine status and codes. list.essentialmeds.org
- StatPearls: Ibuprofen Toxicity — overdose features and management overview. NCBI

I am pursuing MBA in pharmaceutical management from NIPER Hyderabad with a strong academic record and proven success in national-level pharmacy entrance exams. I secured AIR 61 in NIPER 2024 (MS/M.Pharm) and AIR 27 in NIPER MBA, along with AIR 147 in GPAT 2024 and AIR 907 in GPAT 2023. I also achieved AIR 6 in AIIMS CRE-2025 for Drug Store Keeper and was selected as a Pharmacist (AIR 61) for ESIC. Additionally, I was the Runner-Up in Round 2 of the EY Case Study Competition.
At PharmacyFreak.com, I aim to guide future pharmacists through expert content, exam strategies, and insightful resources based on real experience and academic excellence.
Mail- harsh@pharmacyfreak.com