Cancer is characterized by uncontrolled cell growth and the ability of malignant cells to invade other tissues. Anticancer drugs, also called antineoplastic agents, are used to destroy or inhibit the proliferation of cancer cells. These drugs work through a variety of mechanisms and are classified based on their mode of action and chemical structure.
This blog from Pharmacy Freak covers the classification, uses, drug of choice highlights, side effects, and clinical pearls relevant for academic and clinical understanding of anticancer pharmacology.
Table of Contents
What is Anticancer Drug (1)
Anticancer drugs are pharmacological agents used in the treatment of malignant tumors. They act by interfering with cell division or damaging DNA, leading to the death of rapidly dividing cancer cells.
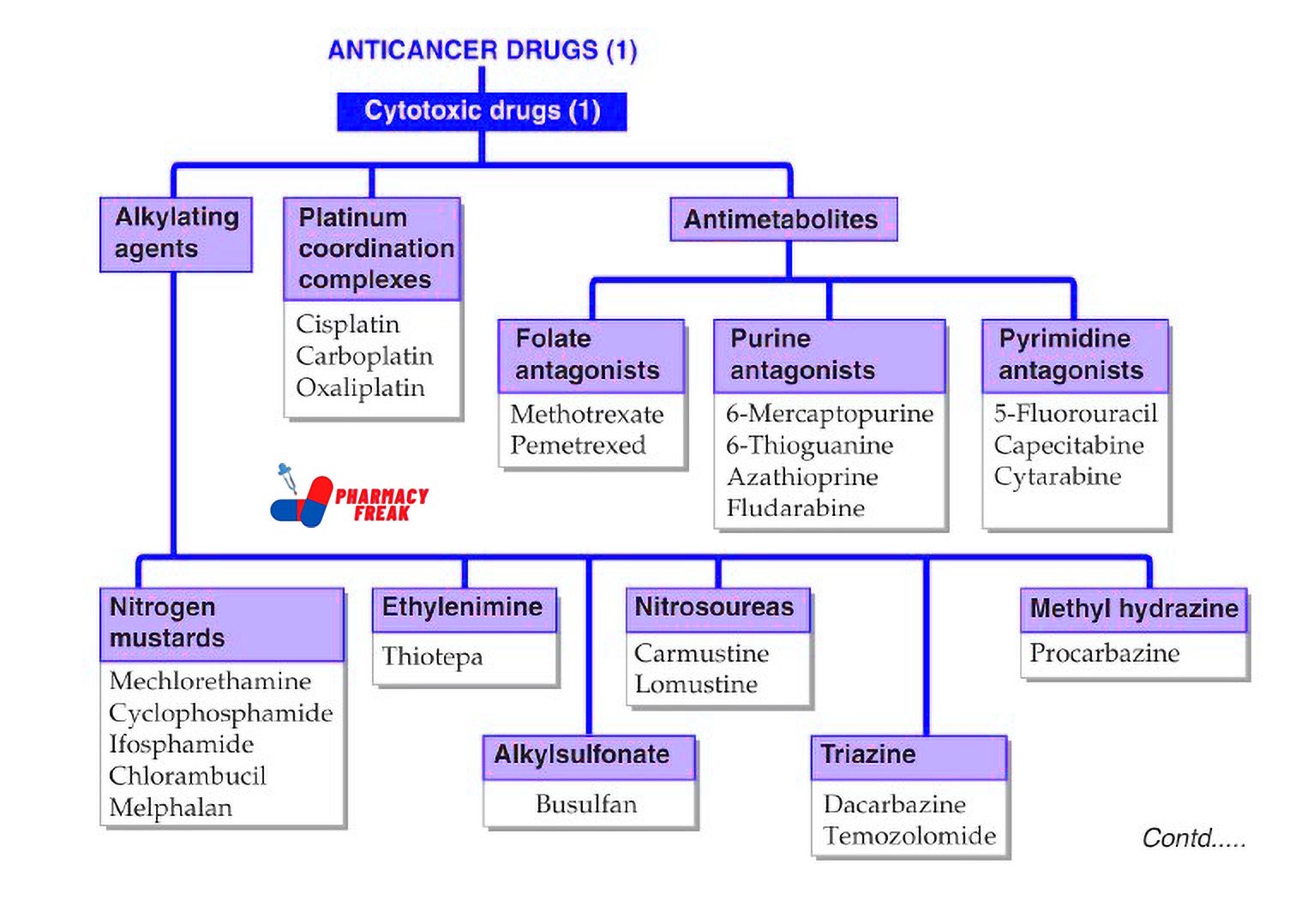
Classification of Anticancer Drugs (KD Tripathi)
ANTICANCER DRUGS (1) – Cytotoxic Drugs (1)
- lkylating agents –
- Nitrogen mustards: Mechlorethamine, Cyclophosphamide, Ifosfamide, Chlorambucil, Melphalan
- Ethylenimine: Thiotepa
- Alkylsulfonate: Busulfan
- Nitrosoureas: Carmustine, Lomustine
- Triazine: Dacarbazine, Temozolomide
- Methyl hydrazine: Procarbazine
- Platinum coordination complexes –
Cisplatin, Carboplatin, Oxaliplatin - Antimetabolites –
- Folate antagonists: Methotrexate, Pemetrexed
- Purine antagonists: 6-Mercaptopurine, 6-Thioguanine, Azathioprine, Fludarabine
- Pyrimidine antagonists: 5-Fluorouracil, Capecitabine, Cytarabine
Classification of Anticancer Drugs (General)
Anticancer drugs are broadly classified into the following categories:
- Alkylating Agents
Mechanism: Form covalent bonds with DNA, leading to cross-linking and inhibition of replication
Examples: Cyclophosphamide, Ifosfamide, Chlorambucil, Busulfan, Melphalan
Use: Broad spectrum – lymphomas, leukemias, breast and ovarian cancers - Platinum Coordination Complexes
Mechanism: Form DNA cross-links similar to alkylating agents
Examples: Cisplatin, Carboplatin, Oxaliplatin
Use: Testicular, ovarian, bladder, and colorectal cancers - Antimetabolites
Mechanism: Inhibit enzymes required for nucleotide synthesis
Subtypes:
- Folate antagonists: Methotrexate
- Purine analogues: 6-Mercaptopurine, 6-Thioguanine
- Pyrimidine analogues: 5-Fluorouracil, Cytarabine, Capecitabine
Use: Leukemias, breast cancer, colorectal cancer
- Plant Alkaloids
a. Vinca alkaloids
Mechanism: Inhibit microtubule assembly
Drugs: Vincristine, Vinblastine
b. Taxanes
Mechanism: Stabilize microtubules, preventing depolymerization
Drugs: Paclitaxel, Docetaxel
c. Epipodophyllotoxins
Mechanism: Inhibit topoisomerase II
Drugs: Etoposide, Teniposide
d. Camptothecins
Mechanism: Inhibit topoisomerase I
Drugs: Topotecan, Irinotecan
Use: Breast cancer, lung cancer, lymphomas, leukemias - Antibiotics (Cytotoxic)
Mechanism: Intercalate into DNA, inhibit topoisomerase II, generate free radicals
Examples: Doxorubicin, Daunorubicin, Bleomycin, Mitomycin C
Use: Leukemias, lymphomas, sarcomas, solid tumors - Hormonal Agents
a. Estrogen antagonists: Tamoxifen (breast cancer)
b. Aromatase inhibitors: Letrozole, Anastrozole
c. Androgen antagonists: Flutamide, Bicalutamide (prostate cancer)
d. GnRH analogues: Leuprolide, Goserelin
Use: Hormone-sensitive cancers - Monoclonal Antibodies
Mechanism: Target specific cell surface antigens
Examples:
- Rituximab (CD20 – B-cell lymphoma)
- Trastuzumab (HER2 – breast cancer)
- Cetuximab (EGFR – colorectal and head-neck cancer)
- Tyrosine Kinase Inhibitors
Mechanism: Inhibit intracellular kinase pathways involved in tumor growth
Examples:
- Imatinib (BCR-ABL in CML)
- Erlotinib, Gefitinib (EGFR)
- Sunitinib, Sorafenib (VEGF receptors)
- Immunomodulators
Examples: Interferons, Interleukins, Thalidomide
Use: Melanoma, renal cell carcinoma, multiple myeloma - Miscellaneous Agents
Examples: Hydroxyurea, Asparaginase, Procarbazine
Use: Chronic myelogenous leukemia, acute lymphoblastic leukemia, Hodgkin’s disease
Uses
Anticancer drugs are used for:
- Curative therapy in hematological malignancies
- Palliative therapy in advanced cancers
- Adjuvant or neoadjuvant therapy in solid tumors
- Prevention of recurrence or metastasis
- Immunotherapy in specific cancers (melanoma, renal cell carcinoma)
Drug of Choice Highlights
- Acute lymphoblastic leukemia – Vincristine + Prednisolone + Asparaginase
- Chronic myeloid leukemia – Imatinib
- Breast cancer – Tamoxifen (ER-positive), Trastuzumab (HER2-positive)
- Prostate cancer – Leuprolide + Flutamide
- Testicular cancer – Cisplatin + Etoposide + Bleomycin
- Hodgkin’s lymphoma – ABVD regimen (Adriamycin, Bleomycin, Vinblastine, Dacarbazine)
- Osteogenic sarcoma – Methotrexate + Doxorubicin + Cisplatin
- Multiple myeloma – Bortezomib + Thalidomide + Dexamethasone
Side Effects
- Alkylating agents – Bone marrow suppression, infertility, secondary malignancies
- Platinum compounds – Nephrotoxicity, ototoxicity, peripheral neuropathy
- Antimetabolites – Myelosuppression, mucositis, hepatotoxicity
- Vinca alkaloids – Neurotoxicity (Vincristine), myelosuppression (Vinblastine)
- Taxanes – Myelosuppression, hypersensitivity reactions
- Anthracyclines – Cardiotoxicity (Doxorubicin)
- Bleomycin – Pulmonary fibrosis
- Hormonal agents – Hot flashes, thromboembolism, osteoporosis
- Monoclonal antibodies – Infusion reactions, cardiotoxicity (Trastuzumab)
- TKIs – Diarrhea, rash, hepatotoxicity, QT prolongation
Updated Clinical Pearls
- Targeted therapies have improved survival in cancers like CML and breast cancer with fewer systemic toxicities.
- Cardiotoxicity with anthracyclines is dose-dependent; cumulative dose monitoring is essential.
- Supportive therapies like antiemetics, colony-stimulating factors, and hydration protocols reduce treatment-related complications.
- Combination chemotherapy is often more effective due to synergistic mechanisms and reduced resistance.
- Monitoring of renal, hepatic, and cardiac function is necessary throughout treatment.
References
- Tripathi KD. Essentials of Medical Pharmacology. 7th ed. New Delhi: Jaypee Brothers Medical Publishers; 2013. p. 829–846
- Gupta S, Garg A. Review of Pharmacology. 15th ed. New Delhi: Jaypee Brothers Medical Publishers; 2023. p. 275–277
- Brunton LL, Chabner BA, Knollmann BC, editors. Goodman and Gilman’s The Pharmacological Basis of Therapeutics. 12th ed. New York: McGraw-Hill Education; 2011. p. 1667–1692
Related Links

I am a Registered Pharmacist under the Pharmacy Act, 1948, and the founder of PharmacyFreak.com. I hold a Bachelor of Pharmacy degree from Rungta College of Pharmaceutical Science and Research. With a strong academic foundation and practical knowledge, I am committed to providing accurate, easy-to-understand content to support pharmacy students and professionals. My aim is to make complex pharmaceutical concepts accessible and useful for real-world application.
Mail- Sachin@pharmacyfreak.com