During reporting an ADR, the healthcare professional should keep in mind that these reports are only showing suspected associations of a drug with a particular adverse event. Reporting an ADR does not confirm a causal relationship between the drug and the adverse reaction. But, it is always better to report a case in a doubtful situation. Any undesirable ADR suspected to be a result of the use of any drug, biological (including blood products), herbal agents, cosmetics, or medical devices should be reported.
Some of the examples are:
- All suspected ADRs to be associated with a prescribed or non-prescribed medication.
- The marketing companies should provide all suspected ADRs regardless of her or not the product was used according to the information provided.
- All the information related to the unexpected reaction should be reported regardless of their nature, severity, and frequency.
- The frequency of a given reaction is observed to be increased.
- Any serious reaction, whether expected or not should be reported.
- All the adverse reactions that may occur due to drug-drug, drug-food, or drug-food supplement interactions should be monitored.
- Monitoring of ADRs in special cases, like drug abuse and drug use in pregnancy and during lactation, should be done properly.
- ADRs due to overdose or medication error should also be reported.
- Reporting of ADRs that result due to unusual lack of efficacy or when suspected pharmaceutical defects are observed.
ADR Case Report
The minimal standard information is required for proper assessment of the ADR reports and it covers the following information:
1) Patient Information
- Patient Identity: It includes initials or record numbers of the patient in the hospital, medical institution, dispensary, clinic, or pharmacy.
- Birth Dates or Age: It includes date, month, and year.
- Sex: Male or female.
- Weight: It should be measured in kilograms.
2) Adverse Reaction(s)
- i) Brief Description of the ADR(s): It gives information about the adverse reaction(s) by marking X on the right box. It gives clear and brief information about the adverse reactions being reported along with the body site and severity.
- ii) Time/Date of Onset of the Adverse Reactions: Clear information should be provided about the time of onset or the occurrence of the adverse reaction concerning the drug administration. The date of onset should be given in the order of day, month, and year. For example, adverse reactions appeared immediately after drug administration or there was a temporal or spatial correlation with administration.
- iii) Other Relevant Information: It includes information about:
- a) Patient’s medical history or laboratory data along with the dates (if available), considered relevant to the case or the adverse reaction being reported should be entered.
- b) Laboratory tests done on the patient and results should be mentioned to confirm the adverse reaction. Such information should be concise and clear.
3) Suspected Drug(s)
- i) Name of the Suspected Drug (s): The trade name of the drug should be mentioned clearly. But in case the trade name is not available, the generic name should be documented along with the strength of the drug(s).
- ii) The dosage, frequency, and administration route of the drug should be mentioned. For example,
- a) Dosage: The dosage form (tablet, capsules, syrup, injection, cream, eye drops, etc.) along with the total amount of drugs should be mentioned.
- b) Frequency: The unit (i.e., mg, ml, mg/kg) and number of times a drug is to be administered (e.g., 4 times daily or q.i.d.) should be mentioned.
- c) Route of Administration: The drug administration route should also be mentioned either in full form or abbreviated form according to the WHO codes.
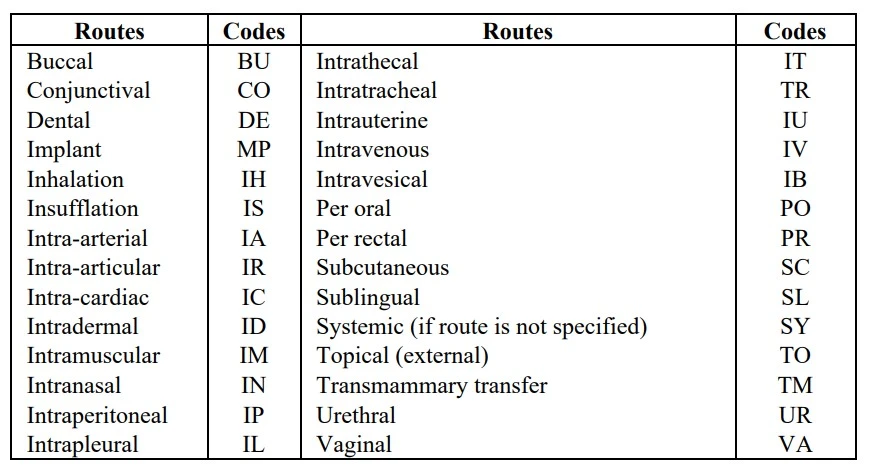
- iii) Therapy Date: The starting and ending date of drug administration should be given clearly in the following manner, i.e., date, month, and year. In case exact dates are not available, the duration of treatment should be mentioned. If drug administration has been continued and not ended at the time of reporting, it should be stated as „Continuing‟.
- iv) Batch Number and Expiry Date: These should be provided if available.
- v) Reason for Use: The condition or disease for which the drug(s) was being administered should be mentioned.
- vi) Particular of Concurrently Drugs (or Other Treatment): Information about the other drug(s) administered by the patient along with the suspected drug should be given. Also, information on the drugs administered at least 1 month back along with the dosage, administration route, duration, and indications should be mentioned. Information related to medical devices used should also be provided.
4) Management of the Adverse Reaction
- i) Confirmation of the ADRs: It includes the procedures required to confirm the suspected adverse reactions. For example,
- a) ADRs confirmed by disappearance on stopping the administration of the drug or reducing the doses.
- b) Patient recovers on withdrawal of the suspected drug(s) if no other drug is withdrawn and no therapy is given.
- c) Recovery includes the withdrawal of drug-causing ADR along with the treatment of ADRs.
- ii) Criteria: The criteria for considering a drug reaction as ADR should be mentioned.
- iii) Treatment: Information about any treatment given to the patient after experiencing the ADRs should be mentioned.
- iv) Outcome: The symptoms of adverse reaction should be mentioned by marking an X in the appropriate box with dates in case of a fatal outcome.
5) Reporter Information
The name, address of the health facility (hospital, institution, dispensary, clinic, company, pharmacy, or maternity home), E-mail address (optional), signature, telephone number, and date of reporting the reaction (indicate date, month, and year) should be mentioned.
Reporting Person
Submission of a report does not mean that a healthcare professional or the drug or the product caused or contributed to the ADR in any way because all reports are considered suspected results. The reporters should keep in mind that any information related to them and the patient’s identities should remain confidential.
It is the professional responsibility of all healthcare professionals practicing in India including specialists, doctors, dentists, pharmacists, nurses, assistant medical officers, clinical officers, pharmaceutical technicians, pharmaceutical assistants, traditional medicine practitioners, and other healthcare providers to provide reports of any case of suspected ADRs. A system should be developed by the manufacturers or product registrants to follow up on ADR within their company and to analyze the impact of notification of significant safety data on their products.
All government hospitals, private hospitals, health centers, dispensaries, private clinics, private pharmacies, and private nursing homes should report all ADR cases about which the patients have complained. A local person should be appointed by the government and private hospitals, health centers, and dispensaries to coordinate the ADRs collection and report within the facility.
It is compulsory to report an ADR to CDSCO even if a precise relationship is not confirmed with the given medication or all the data and facts are not available. Reports from various healthcare providers in different parts of the country should be collected to identify the associations between a particular drug and the ADR. Therefore, all required information for the submission of ADR reports must be obtained and reported through the reporting forms.
Time to Report
Reporting of any suspected ADR should be done immediately as any delay in reporting may lead to wrong and unreliable reporting. Reporting should possibly be done when the patient is still in the health facility as it gives a chance to the reporter to re-question or examine the patient to clear any doubt.
Way to Report
To make a better and more efficient program for ADR monitoring, the reporter should send accurate information.
Following are the ways that make an efficient and accurate reporting of ADRs:
- 1) The report should be prepared on a standardized form (specially designed for reporting ADRs). This reporting form is a self-adhesive postage-paid “red-colored form”.
- 2) The ADRs reporting form (annexed) should be filled when a patient experiences ADR.
- 3) A different form should be used for different patients.
- 4) A duly filled ADR reporting form should be sealed and mailed within three days directly to CDSCO or through other reporting centers for onward transmission to the CDSCO.
- 5) Reports can also be submitted online by going to the CDSCO website or can be sent by e-mail.
- 6) In case of urgency, the ADR reports should be faxed to CDSCO or a related agency.
- 7) Any additional information related to an ADR case that has been reported later can be sent on another ADR form, or communicated by telephone, fax, or e-mail. The information in the follow-up reports should be matched with the original report and for this following information should be provided in the follow-up report:
- i) It is a follow-up information
- ii) Date of the original report
- iii) Patient’s identity
Basic Principles of Efficient Reporting
Efficient reporting is based on the following basic principles:
- 1) The adverse reaction should be reported as early as possible after it occurred.
- 2) If possible, the report should be prepared when the patient is still with the reporter so that the details can be filled in at once on the reporting form.
- 3) Any other factors that may explain the occurrence of adverse events such as any other prescribed drugs, self-medication, herbal products, food, and chemicals should be mentioned. The patient should be asked particularly about other medicines they are taking.
- 4) If any supplementary data is collected later in case the patient develops some other symptoms or if something happens that increases suspicion or seems to exclude the reaction, a supplementary note should be immediately sent using the ADRs reporting form along with the patient identifiers.
- 5) All the ADR reports should have the following four basic points:
- i) An identifiable patient
- ii) A suspected adverse effect
- iii) A named suspected drug(s)
- iv) An identifiable reporter.
- 6) The report should be written clearly in a readable format.
Source of ADR Reporting Form
The ADR reporting form can be obtained free of charge from the following agencies:
- 1) CDSCO offices
- 2) The website of CDSCO, downloaded https://cdsco.gov.in
- 3) Zonal Drug Information Centres
- 4) Regional hospitals, district hospitals, and ADRs local persons in hospitals, health centers, clinics, and dispensaries.

I am a Registered Pharmacist under the Pharmacy Act, 1948, and the founder of PharmacyFreak.com. I hold a Bachelor of Pharmacy degree from Rungta College of Pharmaceutical Science and Research. With a strong academic foundation and practical knowledge, I am committed to providing accurate, easy-to-understand content to support pharmacy students and professionals. My aim is to make complex pharmaceutical concepts accessible and useful for real-world application.
Mail- Sachin@pharmacyfreak.com
