Table of Contents
Introduction
Epinephrine (adrenaline) is an endogenous catecholamine and a potent sympathomimetic agent that acts as a non-selective agonist at both α- and β-adrenergic receptors. It plays a critical physiological role in the “fight-or-flight” response and is widely used clinically in emergency medicine, anesthesia, cardiology, and allergy management. From an exam perspective, epinephrine is a high-yield drug due to its receptor selectivity, dose-dependent actions, and life-saving indications such as anaphylaxis and cardiac arrest.
Mechanism of Action (Step-wise)
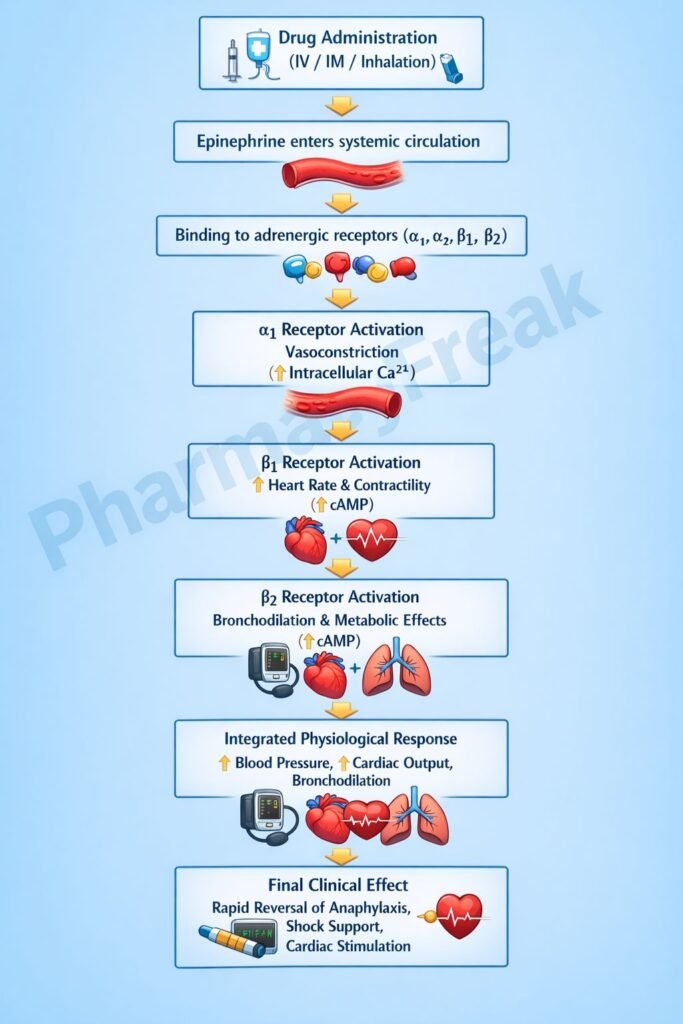
Epinephrine exerts its effects by directly stimulating adrenergic receptors coupled to specific G-proteins.
Step 1: Adrenergic receptor binding
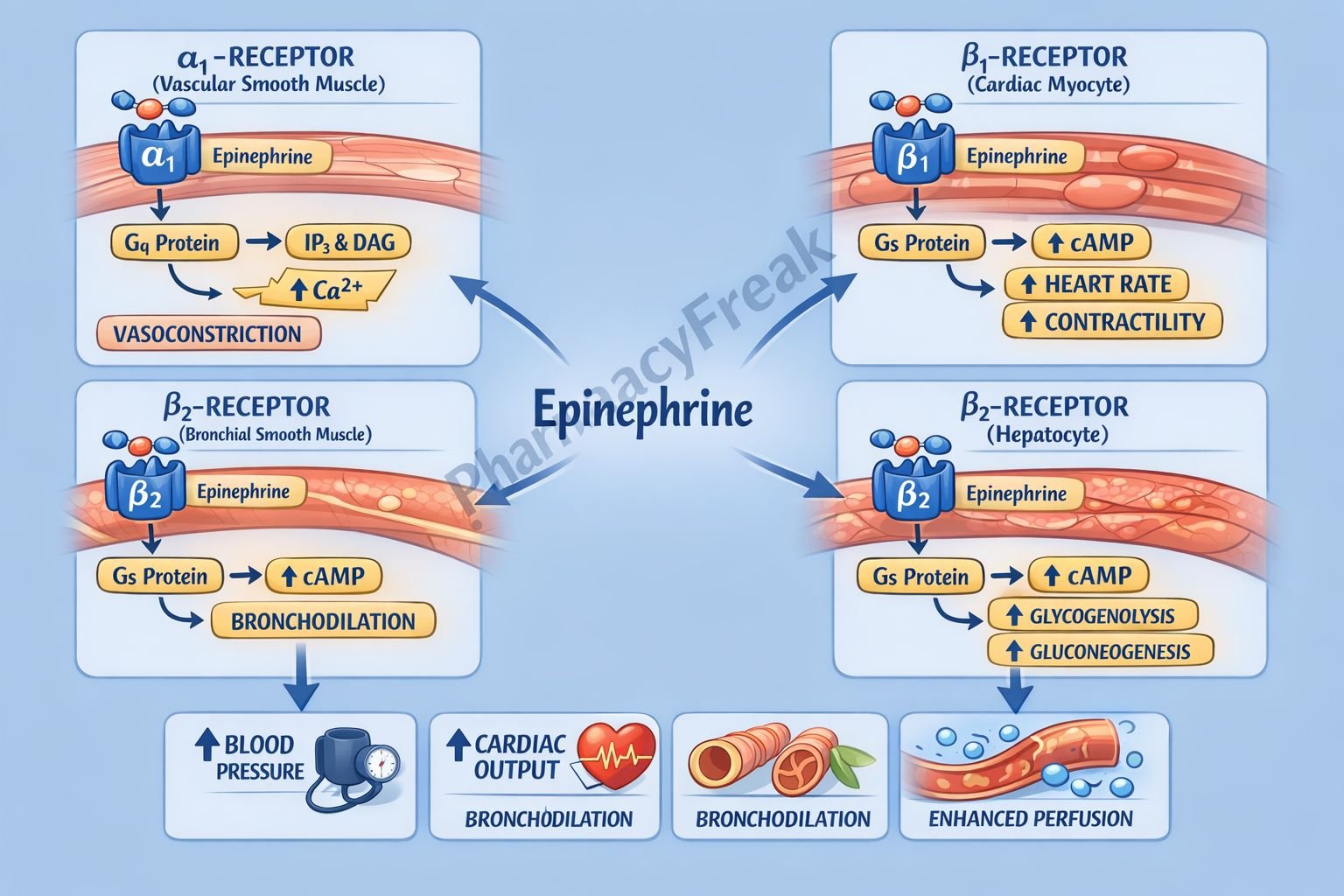
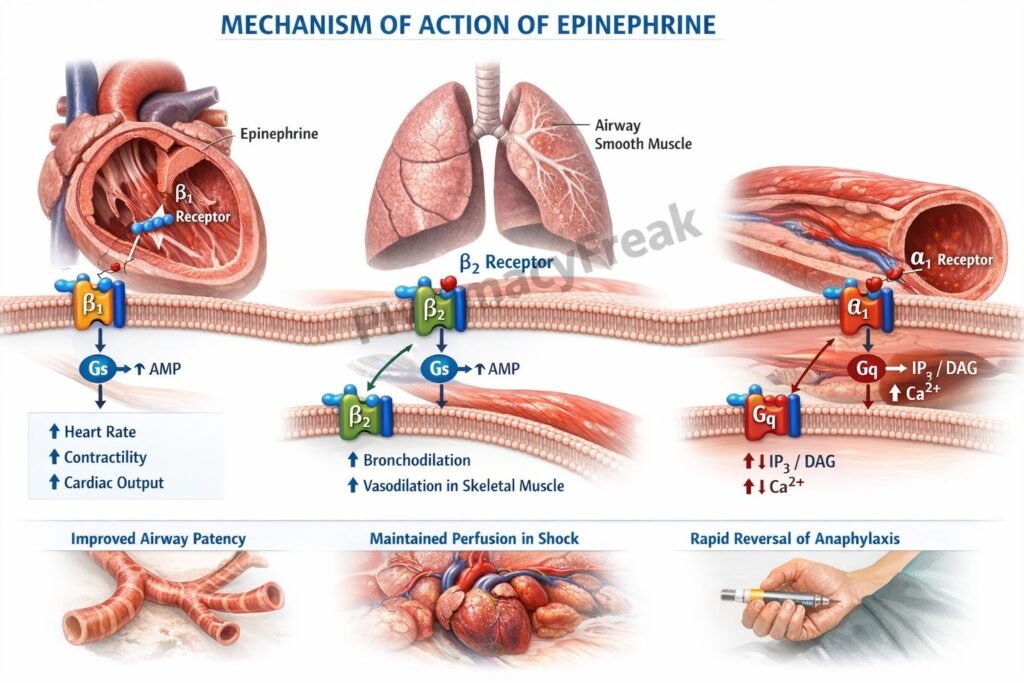
Epinephrine binds to α₁, α₂, β₁, and β₂ adrenergic receptors distributed across cardiovascular, respiratory, and metabolic tissues.
Step 2: α₁-receptor activation (Gq-coupled)
- Activates phospholipase C
- Increases IP₃ and DAG
- Raises intracellular calcium
- Causes vasoconstriction in skin, mucosa, and splanchnic circulation
- Leads to increased peripheral vascular resistance and blood pressure
Step 3: β₁-receptor activation (Gs-coupled)
- Activates adenylate cyclase
- Increases cAMP
- Enhances calcium influx in cardiac myocytes
- Results in positive inotropic, chronotropic, and dromotropic effects
- Increases cardiac output
Step 4: β₂-receptor activation (Gs-coupled)
- Increases cAMP in smooth muscle
- Causes bronchodilation
- Produces vasodilation in skeletal muscle beds
- Relaxes uterine smooth muscle
Step 5: Metabolic effects
- Stimulates glycogenolysis in liver and muscle
- Promotes lipolysis via hormone-sensitive lipase
- Increases blood glucose and free fatty acids
Dose-dependent effect (exam favorite):
- Low dose: β-effects predominate (β₁, β₂)
- High dose: α₁-mediated vasoconstriction becomes dominant
Pharmacokinetics
- Route of administration: IM, IV, SC, inhalational, topical
- Oral bioavailability: Ineffective (extensive first-pass metabolism)
- Onset of action: Rapid (especially IV and IM)
- Distribution: Widely distributed; poor CNS penetration
- Metabolism: Rapidly metabolized by COMT and MAO in liver and other tissues
- Half-life: Very short (2–3 minutes)
- Excretion: Metabolites excreted in urine (vanillylmandelic acid, metanephrines)
Clinical Uses
- Anaphylaxis (drug of choice) – reverses bronchospasm, hypotension, and laryngeal edema
- Cardiac arrest – improves coronary and cerebral perfusion during CPR
- Acute severe asthma – bronchodilation via β₂ stimulation
- Adjunct to local anesthetics – prolongs action by vasoconstriction
- Hypotension and shock – increases systemic vascular resistance and cardiac output
- Open-angle glaucoma (topical, rarely used) – reduces aqueous humor production
Adverse Effects
Cardiovascular:
- Tachycardia
- Palpitations
- Hypertension
- Cardiac arrhythmias
- Angina
Central nervous system:
- Anxiety
- Tremor
- Headache
- Restlessness
Metabolic:
- Hyperglycemia
- Hypokalemia
Local effects:
- Tissue necrosis with extravasation (α₁-mediated vasoconstriction)
Comparative Analysis
Epinephrine vs Norepinephrine vs Isoproterenol
| Feature | Epinephrine | Norepinephrine | Isoproterenol |
|---|---|---|---|
| α₁ activity | Strong | Very strong | Absent |
| β₁ activity | Strong | Moderate | Very strong |
| β₂ activity | Strong | Weak | Very strong |
| Effect on BP | Biphasic | Marked increase | Decrease |
| Bronchodilation | Present | Minimal | Marked |
| Use in anaphylaxis | Yes | No | No |
Explanation:
Epinephrine’s unique combination of α and β activity makes it ideal for anaphylaxis, where both vasoconstriction and bronchodilation are required. Norepinephrine lacks significant β₂ activity, while isoproterenol lacks α activity, limiting their utility in allergic emergencies.
MCQs
- Epinephrine increases intracellular calcium in cardiac muscle primarily via:
a) Gi protein
b) Gq protein
c) Gs protein
d) Ion channel blockade
Answer: c) Gs protein
- The bronchodilator effect of epinephrine is mediated by:
a) α₁ receptors
b) α₂ receptors
c) β₁ receptors
d) β₂ receptors
Answer: d) β₂ receptors
- At low doses, epinephrine predominantly causes:
a) Vasoconstriction
b) Bradycardia
c) Vasodilation in skeletal muscle
d) Reduced cardiac output
Answer: c) Vasodilation in skeletal muscle
- Epinephrine is inactivated mainly by:
a) CYP450
b) Esterases
c) COMT and MAO
d) Renal excretion unchanged
Answer: c) COMT and MAO
- Drug of choice for anaphylactic shock is:
a) Dopamine
b) Norepinephrine
c) Epinephrine
d) Phenylephrine
Answer: c) Epinephrine
- Epinephrine prolongs local anesthetic action by:
a) Reducing metabolism
b) Blocking sodium channels
c) Causing vasoconstriction
d) Increasing lipid solubility
Answer: c) Causing vasoconstriction
- Which receptor mediates epinephrine-induced glycogenolysis?
a) α₁
b) α₂
c) β₁
d) β₂
Answer: d) β₂
- Short half-life of epinephrine is due to:
a) Renal clearance
b) Plasma protein binding
c) Rapid enzymatic degradation
d) Poor tissue distribution
Answer: c) Rapid enzymatic degradation
- Epinephrine causes increased cardiac output mainly by:
a) Reduced afterload
b) Increased preload
c) Positive inotropic and chronotropic effects
d) Venodilation
Answer: c) Positive inotropic and chronotropic effects
FAQs
1. Why is epinephrine preferred in anaphylaxis?
Because it provides bronchodilation, vasoconstriction, and cardiac stimulation simultaneously.
2. Why is epinephrine not effective orally?
It undergoes extensive first-pass metabolism by COMT and MAO.
3. What explains the biphasic blood pressure response?
β₂-mediated vasodilation at low doses and α₁-mediated vasoconstriction at high doses.
4. Can epinephrine cross the blood–brain barrier?
No, due to its polar catechol structure.
5. Why does extravasation cause tissue necrosis?
Intense α₁-mediated vasoconstriction reduces local blood flow.
References
- Goodman & Gilman’s The Pharmacological Basis of Therapeutics
https://accesspharmacy.mhmedical.com - Katzung BG. Basic and Clinical Pharmacology
https://accessmedicine.mhmedical.com - Tripathi KD. Essentials of Medical Pharmacology
- Harrison’s Principles of Internal Medicine
https://accessmedicine.mhmedical.com

I am pursuing MBA in pharmaceutical management from NIPER Hyderabad with a strong academic record and proven success in national-level pharmacy entrance exams. I secured AIR 61 in NIPER 2024 (MS/M.Pharm) and AIR 27 in NIPER MBA, along with AIR 147 in GPAT 2024 and AIR 907 in GPAT 2023. I also achieved AIR 6 in AIIMS CRE-2025 for Drug Store Keeper and was selected as a Pharmacist (AIR 61) for ESIC. Additionally, I was the Runner-Up in Round 2 of the EY Case Study Competition.
At PharmacyFreak.com, I aim to guide future pharmacists through expert content, exam strategies, and insightful resources based on real experience and academic excellence.
Mail- harsh@pharmacyfreak.com