Table of Contents
1. Identification
Summary
Amlodipine is a dihydropyridine calcium-channel blocker for hypertension and chronic stable/vasospastic angina; long half-life supports once-daily dosing.
Brand Names
Norvasc; multiple generics
Name
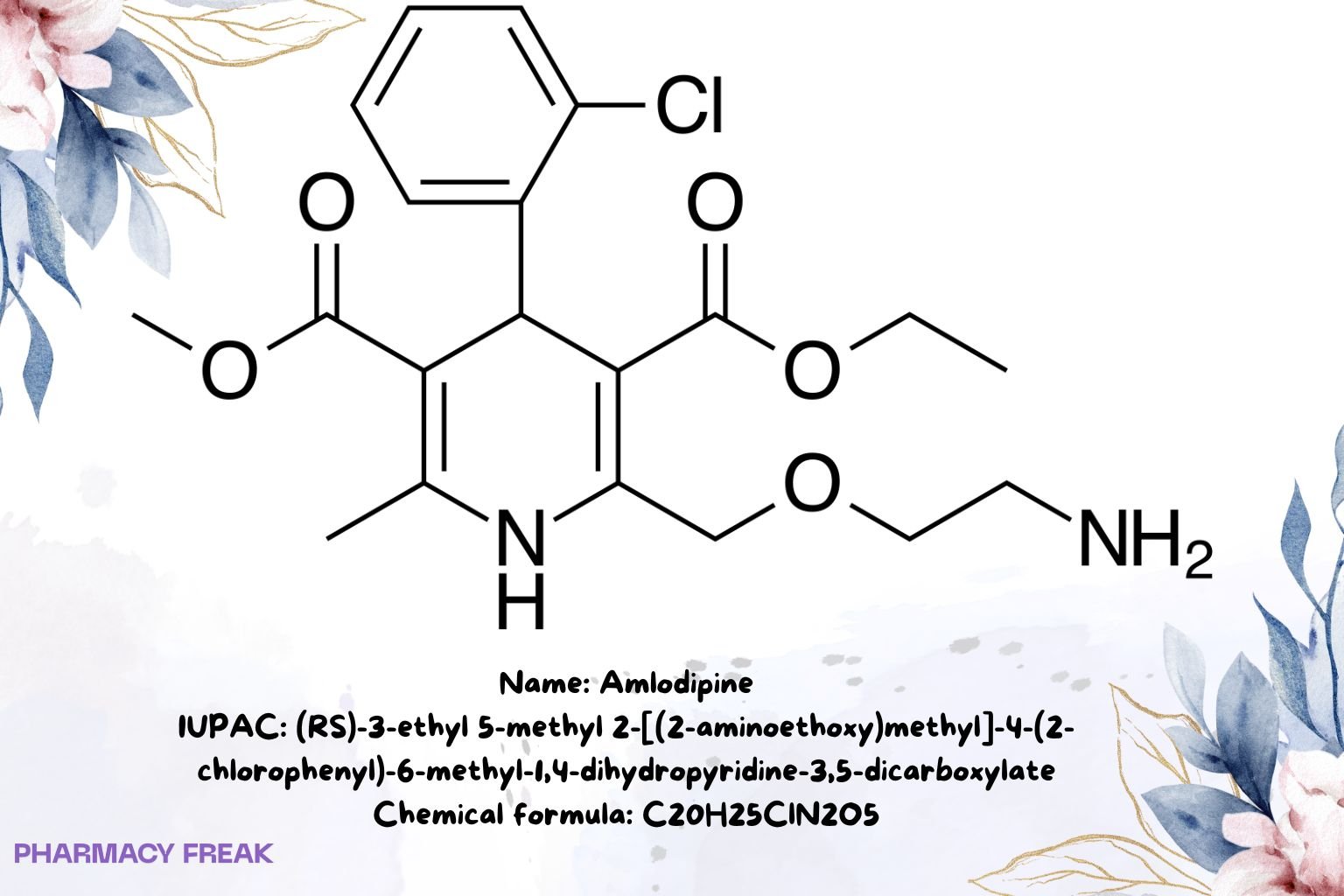
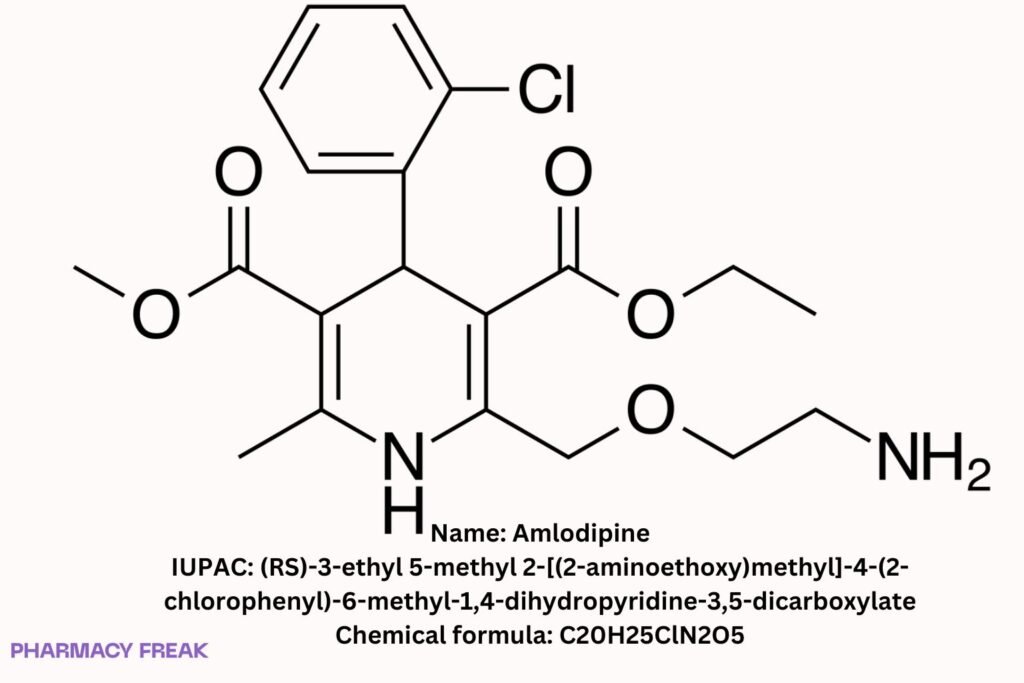
Amlodipine
Background
Racemic, lipophilic dihydropyridine; marketed clinically as amlodipine besylate; oral tablets and solutions dominate.
Modality
Small molecule
Groups
Approved; prescription
Structure
Weight
≈ 408.88 g/mol (free base)
Chemical Formula
C₂₀H₂₅ClN₂O₅ (free base)
Synonyms
(RS)-3-ethyl 5-methyl 2-[(2-aminoethoxy)methyl]-4-(2-chlorophenyl)-6-methyl-1,4-dihydropyridine-3,5-dicarboxylate
External IDs
CAS (base): 88150-42-9; PubChem CID: 2162; UNII (base): 1J444QC288; ATC: C08CA01
2. Pharmacology
Indication
Hypertension; chronic stable angina; vasospastic (Prinzmetal) angina.
Associated Conditions
CAD without heart failure; pediatric hypertension (≥6 years) per labels.
Associated Therapies
Fixed combinations with ARBs/ACEIs; co-therapy with statins and other antihypertensives as needed.
Contraindications & Blackbox Warnings
No boxed warning; contraindicated in known hypersensitivity. Dose cautiously in hepatic impairment.
Pharmacodynamics
Selective L-type Ca²⁺ channel blockade in vascular smooth muscle → arteriolar vasodilation → ↓ SVR and BP; minimal direct cardiac suppression at therapeutic dose.
Mechanism of action
Dihydropyridine ring binds L-type channels in the inactivated state, reducing Ca²⁺ influx and tone.
Absorption
Oral bioavailability ~64–90%; tₘₐₓ 6–12 h; food does not meaningfully alter exposure.
Volume of distribution
~21 L/kg (large, extensive tissue distribution).
Protein binding
~93–98%.
Metabolism
Extensive hepatic metabolism (primarily CYP3A4) to inactive metabolites.
Route of elimination
~60% of dose recovered in urine as metabolites; ~10% unchanged; fecal remainder.
Half-life
~30–50 h (longer in hepatic impairment).
Clearance
Hepatic; exposure increases with strong CYP3A inhibition.
Adverse Effects
Peripheral edema, dizziness, flushing, palpitations; uncommon hypotension; rare serious hepatic or dermatologic events.
Toxicity
Overdose → profound vasodilation and hypotension; supportive care and vasopressors as indicated.
Pathways
Calcium influx inhibition (L-type); hemodynamic afterload reduction.
Pharmacogenomic Effects/ADRs
No routine PGx; transporter/enzyme variants (e.g., CYP3A, CYP3A5) may modulate exposure.
3. Interactions
Drug Interactions
Strong CYP3A inhibitors (e.g., clarithromycin, azoles, protease inhibitors) ↑ amlodipine levels; monitor dose. Simvastatin exposure increases—limit simvastatin to ≤20 mg/day when co-administered. Minimal effect on binding of digoxin/warfarin/phenytoin/indomethacin in vitro.
Food Interactions
Food: no relevant effect. Grapefruit juice: no significant effect on amlodipine PK at tested amounts.
4. Categories
ATC Codes
C08CA01 (amlodipine, dihydropyridine derivatives)
Drug Categories
Calcium-channel blocker; Antihypertensive; Antianginal; Small molecule
Chemical Taxonomy
1,4-dihydropyridine with 2-chlorophenyl substituent; diester at 3,5-positions; tertiary amine side chain.
Affected organisms
Humans (therapeutic use)
5. Chemical Identifiers
UNII
1J444QC288 (amlodipine, base)
CAS number
88150-42-9 (base)
InChI Key
HTIQEAQVCYTUBX-UHFFFAOYSA-N
InChI
InChI=1S/C20H25ClN2O5/c1-4-28-20(25)18-15(11-27-10-9-22)23-12(2)16(19(24)26-3)17(18)13-7-5-6-8-14(13)21/h5-8,17,23H,4,9-11,22H2,1-3H3
IUPAC Name
(RS)-3-ethyl 5-methyl 2-[(2-aminoethoxy)methyl]-4-(2-chlorophenyl)-6-methyl-1,4-dihydropyridine-3,5-dicarboxylate
SMILES
Clc1ccccc1C2/C(C(=O)OC)=C(/C)N/C(COCCN)=C2/C(=O)OCC
6. References
DailyMed/Norvasc labels: bioavailability 64–90%; tₘₐₓ 6–12 h; protein binding ~93%; renal recovery 10% unchanged; half-life 30–50 h; grapefruit juice no significant effect. DailyMed+4DailyMed+4DailyMed+4
Pfizer Norvasc label PDF: Vd ≈21 L/kg; protein binding ≈97.5%; food no effect. Pfizer Labeling
Clinical PK literature: Vd ~21 L/kg; high protein binding ~98%; clearance values in adults. FDA Access Data+3PubMed+3SpringerLink+3
PubChem: identifiers (CID 2162), formula C₂₀H₂₅ClN₂O₅, canonical InChI/SMILES. PubChem
CAS Common Chemistry: CAS 88150-42-9; InChIKey HTIQEAQVCYTUBX-UHFFFAOYSA-N. Common Chemistry
FDA GSRS/UNII: 1J444QC288; formula; InChIKey. precisionFDA
WHOCC ATC/DDD Index: C08CA01 classification. FHI

I am pursuing MBA in pharmaceutical management from NIPER Hyderabad with a strong academic record and proven success in national-level pharmacy entrance exams. I secured AIR 61 in NIPER 2024 (MS/M.Pharm) and AIR 27 in NIPER MBA, along with AIR 147 in GPAT 2024 and AIR 907 in GPAT 2023. I also achieved AIR 6 in AIIMS CRE-2025 for Drug Store Keeper and was selected as a Pharmacist (AIR 61) for ESIC. Additionally, I was the Runner-Up in Round 2 of the EY Case Study Competition.
At PharmacyFreak.com, I aim to guide future pharmacists through expert content, exam strategies, and insightful resources based on real experience and academic excellence.
Mail- harsh@pharmacyfreak.com