Table of Contents
Introduction
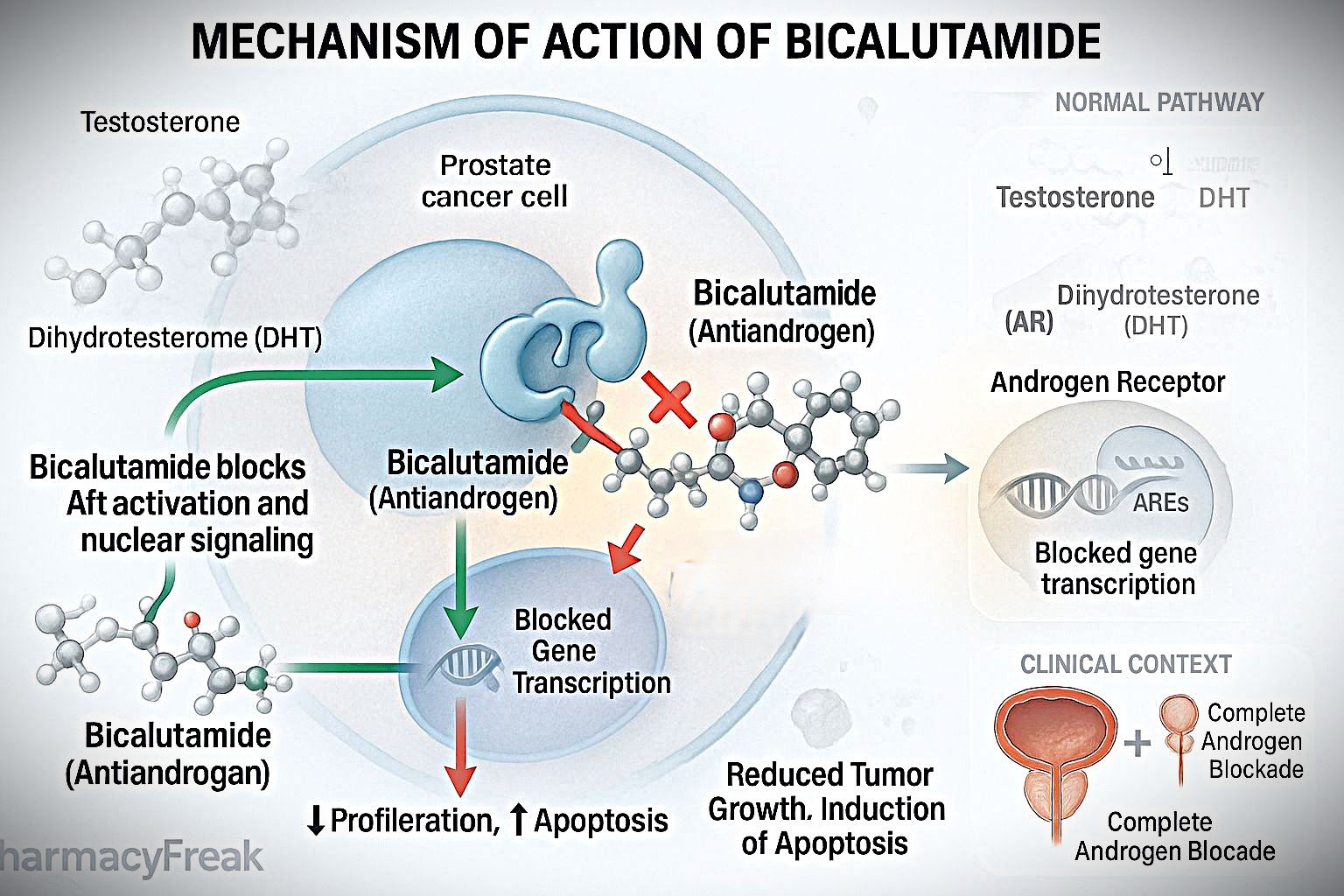
Bicalutamide is a non-steroidal antiandrogen used primarily in the treatment of prostate cancer. It acts by blocking the action of androgens, which are critical in the development and progression of prostate malignancies. Unlike steroidal antiandrogens, bicalutamide offers selective androgen receptor antagonism with fewer hormonal side effects, making it an important drug in androgen deprivation therapy (ADT).
Mechanism of Action (Step-wise)
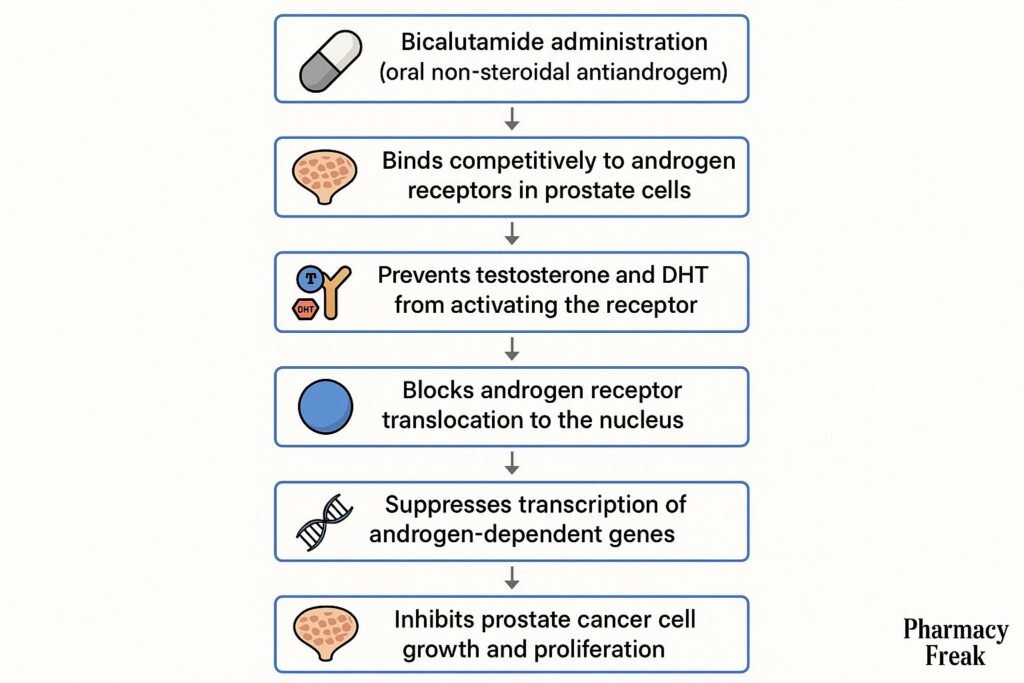
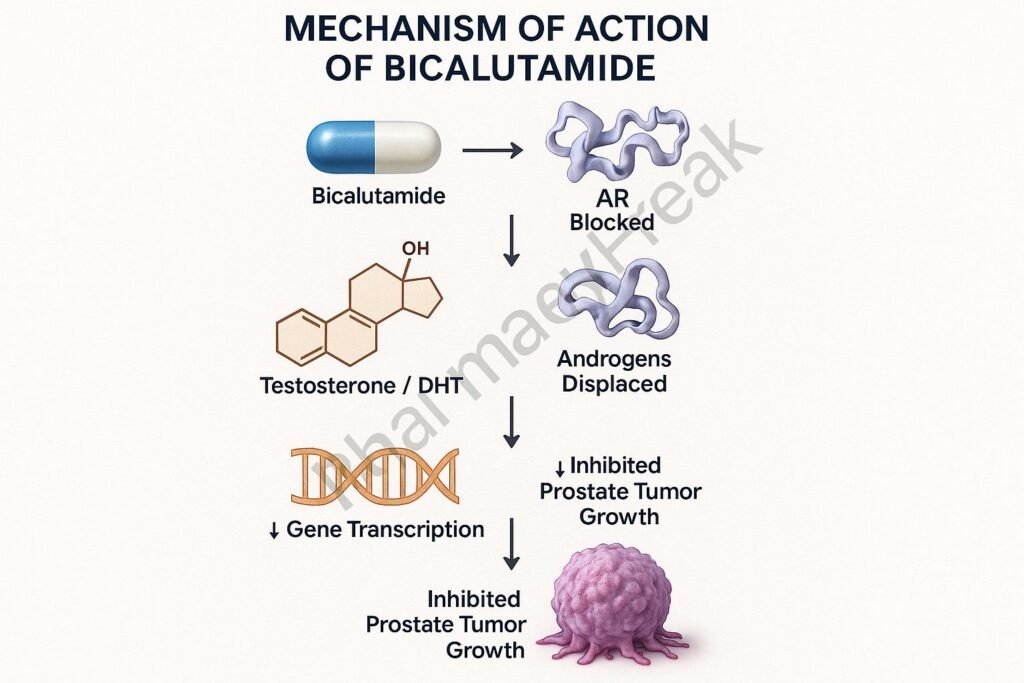
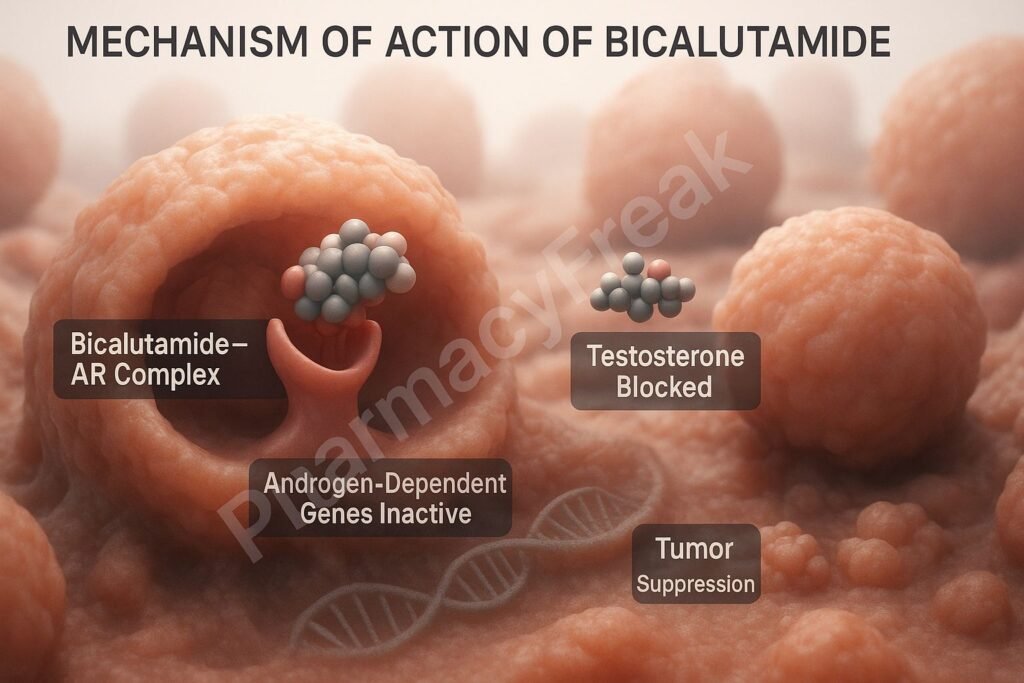
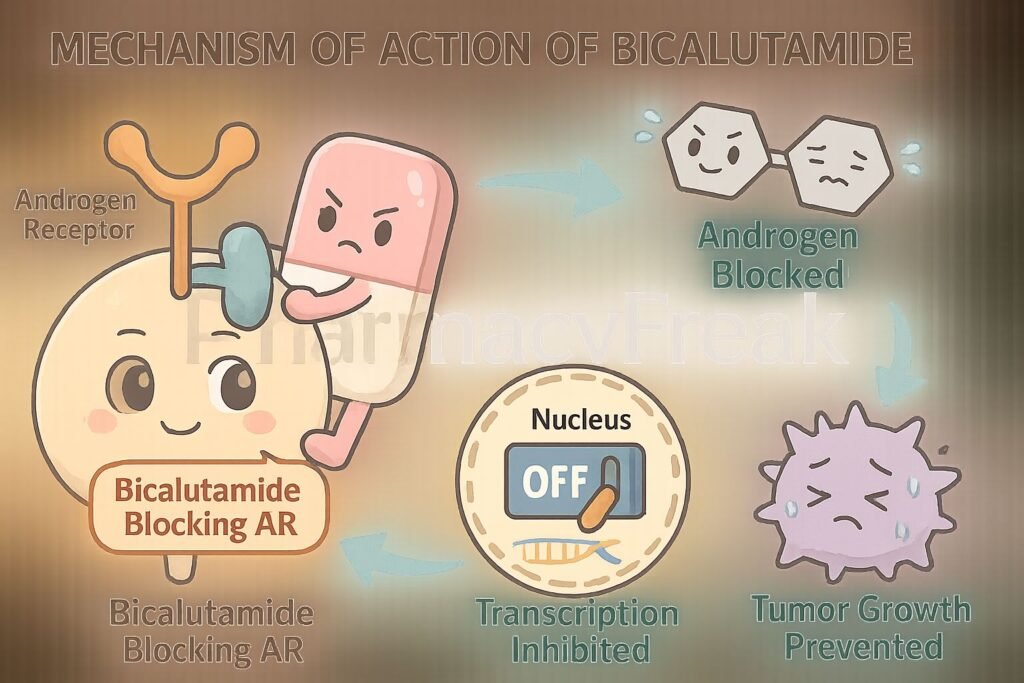
1. Competitive Binding to Androgen Receptors
Bicalutamide binds to cytoplasmic androgen receptors in target tissues like the prostate.
2. Receptor Blockade
By occupying these receptors, it prevents natural androgens (like testosterone and dihydrotestosterone) from binding.
3. Inhibition of AR Translocation
The drug-receptor complex does not translocate to the nucleus, preventing activation of androgen-responsive genes.
4. Suppression of Tumor Growth Signals
This leads to reduced expression of genes involved in prostate cell proliferation and survival.
5. Clinical Antiandrogen Effect
The outcome is inhibition of tumor progression in androgen-dependent prostate cancer.
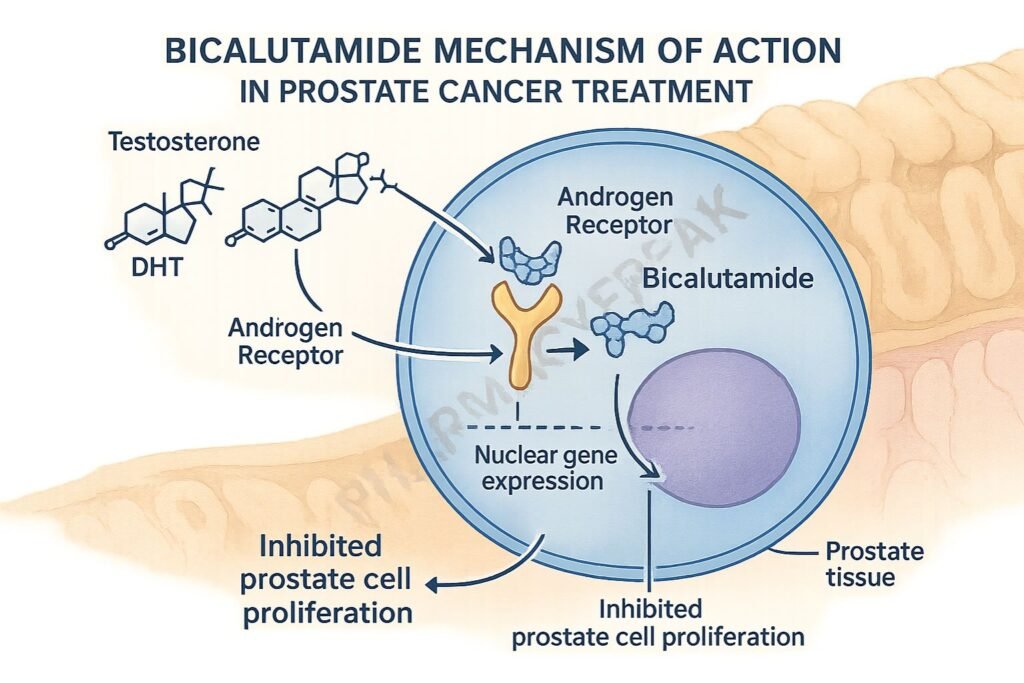

Pharmacokinetics
- Route of Administration: Oral
- Bioavailability: Nearly 100%
- Time to Peak Plasma: 30–40 hours
- Half-Life: ~6 days
- Protein Binding: >95%
- Metabolism: Hepatic (CYP3A4)
- Excretion: Fecal and renal
Clinical Uses
- Prostate cancer (especially advanced/metastatic, often with GnRH agonists)
- Off-label: Hirsutism and other androgen-mediated disorders in females (rare)
Adverse Effects
- Gynecomastia and breast tenderness
- Hot flashes
- Decreased libido
- Hepatotoxicity (rare but serious)
- Gastrointestinal upset
- Anemia and fatigue
- Contraindications: Pregnancy, severe hepatic impairment
Comparative Analysis
| Parameter | Bicalutamide | Flutamide |
|---|---|---|
| Drug Class | Non-steroidal antiandrogen | Non-steroidal antiandrogen |
| Half-Life | ~6 days | ~8 hours |
| Hepatotoxicity Risk | Low to moderate | Higher |
| Dosage Frequency | Once daily | Multiple times daily |
| Preferred in ADT | Yes | Less preferred |
Multiple Choice Questions (MCQs)
1. What receptor does bicalutamide primarily antagonize?
a) Estrogen receptor
b) Androgen receptor
c) GnRH receptor
d) Progesterone receptor
Answer: b) Androgen receptor
2. Bicalutamide is primarily used in the treatment of:
a) Breast cancer
b) Ovarian cancer
c) Prostate cancer
d) Testicular cancer
Answer: c) Prostate cancer
3. The half-life of bicalutamide is approximately:
a) 6 hours
b) 12 hours
c) 2 days
d) 6 days
Answer: d) 6 days
4. A notable side effect of bicalutamide therapy is:
a) Weight gain
b) Gynecomastia
c) Bradycardia
d) Hyperglycemia
Answer: b) Gynecomastia
5. What is the route of excretion for bicalutamide?
a) Renal only
b) Fecal only
c) Both renal and fecal
d) Pulmonary
Answer: c) Both renal and fecal
FAQs
Q1. Is bicalutamide used alone or in combination?
Usually used with GnRH agonists for complete androgen blockade.
Q2. How long does it take to see effects?
Therapeutic effects are observed over weeks to months.
Q3. Can women take bicalutamide?
It’s rarely used off-label for female androgen-related conditions but is contraindicated in pregnancy.
Q4. Does bicalutamide affect testosterone levels directly?
No, it blocks androgen receptors without altering testosterone production.
Q5. Is liver function monitoring necessary?
Yes, periodic liver enzyme checks are recommended due to potential hepatotoxicity.
References
- Goodman & Gilman’s The Pharmacological Basis of Therapeutics, 12th Edition
- KD Tripathi, Essentials of Medical Pharmacology, 7th Edition
- Prescribing information for Bicalutamide
- Clinical practice guidelines for prostate cancer treatment
Related Internal Links

I am pursuing MBA in pharmaceutical management from NIPER Hyderabad with a strong academic record and proven success in national-level pharmacy entrance exams. I secured AIR 61 in NIPER 2024 (MS/M.Pharm) and AIR 27 in NIPER MBA, along with AIR 147 in GPAT 2024 and AIR 907 in GPAT 2023. I also achieved AIR 6 in AIIMS CRE-2025 for Drug Store Keeper and was selected as a Pharmacist (AIR 61) for ESIC. Additionally, I was the Runner-Up in Round 2 of the EY Case Study Competition.
At PharmacyFreak.com, I aim to guide future pharmacists through expert content, exam strategies, and insightful resources based on real experience and academic excellence.
Mail- harsh@pharmacyfreak.com