Table of Contents
Introduction
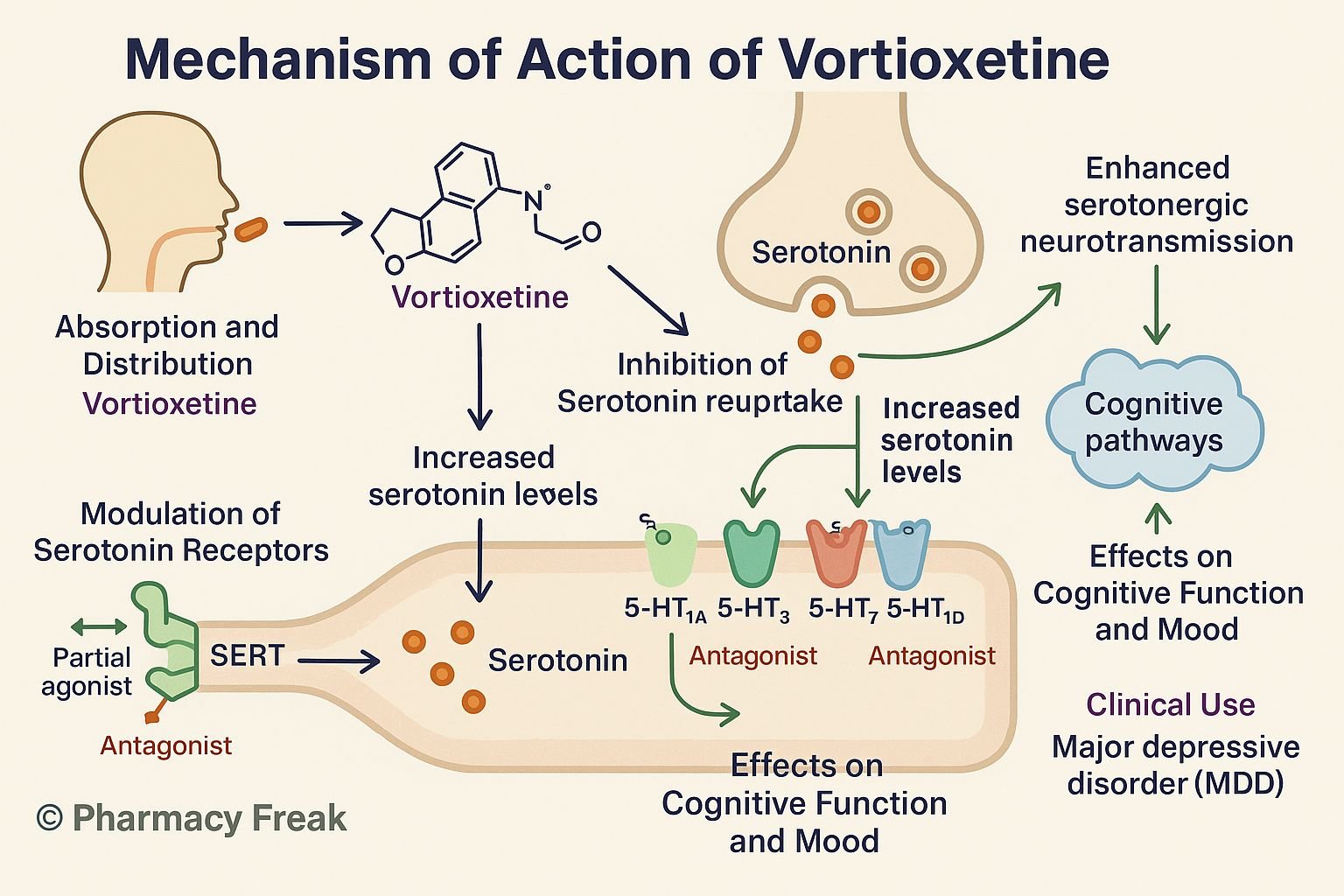
Vortioxetine is a multimodal antidepressant approved for major depressive disorder (MDD). It uniquely combines serotonin transporter (SERT) inhibition with agonist, partial agonist, and antagonist actions at various serotonin receptors, enhancing efficacy on mood, cognition, and anxiety.
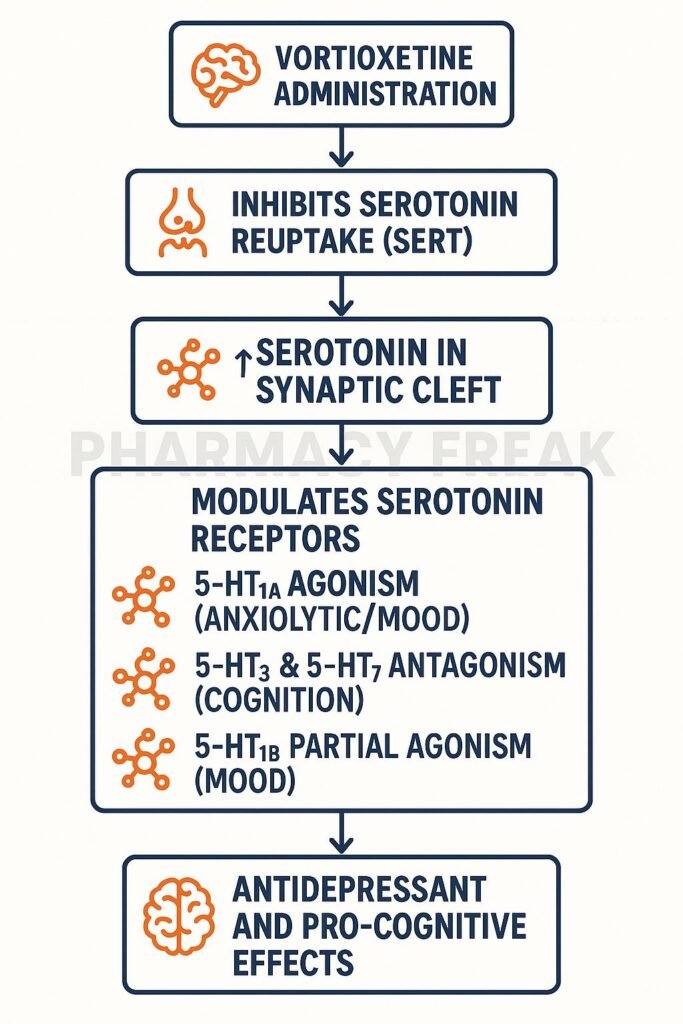
Step-by-Step Mechanism of Action
- Inhibition of SERT
Vortioxetine blocks the serotonin transporter, increasing extracellular serotonin levels. - Direct receptor modulation
- 5‑HT₁A receptor agonist: Enhances serotonergic tone and reduces anxiety
- 5‑HT₁B partial agonist: Regulates serotonin release
- 5‑HT₃ receptor antagonist: Reduces gastrointestinal side effects and modulates cortex function
- 5‑HT₇ receptor antagonist: Improves cognitive processing and circadian modulation
- Enhanced serotonergic neurotransmission
Combined effects on transporter and receptors improve mood, anxiety, and cognitive domains. - Downstream neuroplastic effects
Increased BDNF release and hippocampal neurogenesis contribute to sustained antidepressant effects. - Multimodal synergy
Unique combination may provide faster cognitive improvement, fewer GI side effects, and better overall tolerability.
Pharmacokinetic Parameters
| Parameter | Value |
|---|---|
| Route | Oral tablet, once daily |
| Bioavailability | ~75–90% |
| Time to Peak (Tmax) | ~7–11 hours |
| Protein Binding | ~98–99% |
| Metabolism | CYP2D6 (major) and CYP3A4 (minor) |
| Half-life | ~66 hours |
| Excretion | Metabolites in urine and feces |
Clinical Uses
- Major depressive disorder (MDD)
- Off-label use: generalized anxiety, cognitive symptoms in depression
Adverse Effects
- Nausea (most common)
- Sexual dysfunction
- Headache and dizziness
- Rare: hyponatremia, hypersensitivity
- Low discontinuation rates due to mild side effect profile
Comparative Analysis
| Agent | Mood Effect | Cognitive Benefit | Key Side Effects |
|---|---|---|---|
| Vortioxetine | Yes | Significant | Low GI, mild sexual issues |
| Sertraline | Yes | Mild | High GI, sexual dysfunction |
| Duloxetine | Yes | Mild–moderate | Moderate GI, hypertension |
MCQs
- Vortioxetine’s action includes:
a) SERT inhibition only
b) Receptor modulation only
c) Both SERT inhibition and receptor modulation
d) Dopamine transporter blockade
Answer: c) Both SERT inhibition and receptor modulation - It acts as an agonist at which receptor?
a) 5‑HT₁A
b) 5‑HT₃
c) 5‑HT₇
d) 5‑HT₂C
Answer: a) 5‑HT₁A - Receptor antagonism at 5‑HT₃ leads to:
a) Enhanced GI side effects
b) Fewer GI side effects
c) More anxiety
d) Worse cognition
Answer: b) Fewer GI side effects - Which metabolism pathway is primary?
a) CYP3A4
b) CYP2D6
c) UGT
d) Renal excretion
Answer: b) CYP2D6 - Half-life is approximately:
a) 8 hours
b) 24 hours
c) 66 hours
d) 7 days
Answer: c) 66 hours - Cognitive benefit compared to sertraline is:
a) Similar
b) Superior
c) Inferior
d) None
Answer: b) Superior - Protein binding is around:
a) 50%
b) 75%
c) >98%
d) 100%
Answer: c) >98% - Receptor partial agonism occurs at:
a) 5‑HT₃
b) 5‑HT₁B
c) 5‑HT₇
d) 5‑HT₂A
Answer: b) 5‑HT₁B - Compared to duloxetine, vortioxetine side effects are:
a) More severe
b) Similar
c) Milder
d) Unknown
Answer: c) Milder - Neuroplastic effects include:
a) Dendritic pruning
b) BDNF increase
c) Dopamine decrease
d) NMDA blockade
Answer: b) BDNF increase
FAQs
1. Does vortioxetine improve cognition?
Yes—studies show enhanced executive function and processing speed.
2. Is sexual dysfunction common?
Less common compared to SSRIs and SNRIs, but possible.
3. Can it cause serotonin syndrome?
Rarely, if combined with other serotonergic agents like MAO inhibitors or triptans.
4. Is dose adjustment needed in liver impairment?
Yes—lower dose (5 mg) is recommended in moderate hepatic impairment.
5. How long before effects manifest?
Some patients report mood improvement within 1–2 weeks; full benefit often by 6–8 weeks.
References

I am pursuing MBA in pharmaceutical management from NIPER Hyderabad with a strong academic record and proven success in national-level pharmacy entrance exams. I secured AIR 61 in NIPER 2024 (MS/M.Pharm) and AIR 27 in NIPER MBA, along with AIR 147 in GPAT 2024 and AIR 907 in GPAT 2023. I also achieved AIR 6 in AIIMS CRE-2025 for Drug Store Keeper and was selected as a Pharmacist (AIR 61) for ESIC. Additionally, I was the Runner-Up in Round 2 of the EY Case Study Competition.
At PharmacyFreak.com, I aim to guide future pharmacists through expert content, exam strategies, and insightful resources based on real experience and academic excellence.
Mail- harsh@pharmacyfreak.com