Table of Contents
Introduction
Lacosamide is a third-generation antiepileptic drug (AED) that is primarily used in the treatment of partial-onset seizures. It represents a novel approach to seizure control by selectively enhancing the slow inactivation of voltage-gated sodium channels. This distinct mechanism differentiates it from older AEDs, offering better tolerability and fewer drug interactions.
Mechanism of Action (Step-wise)
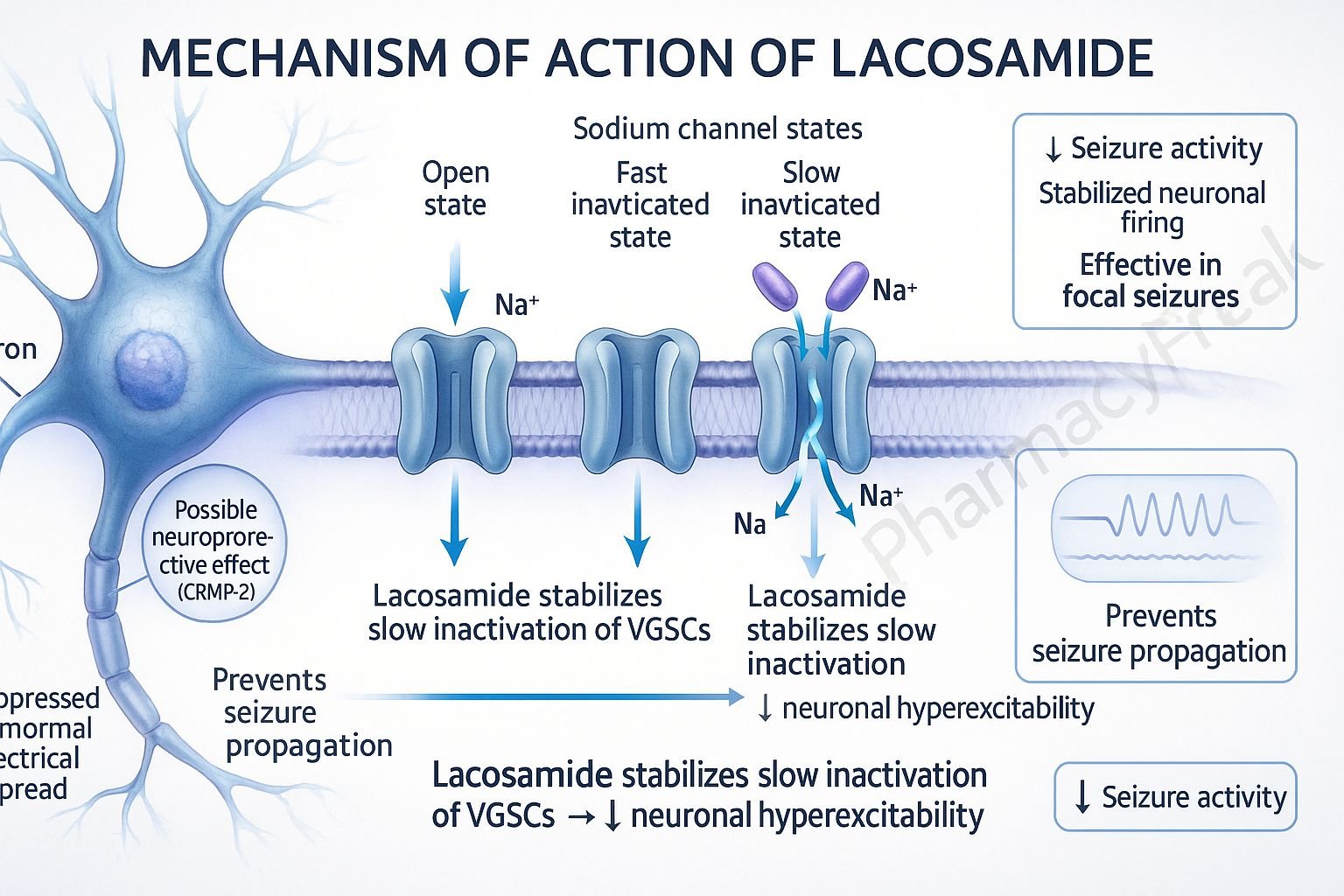
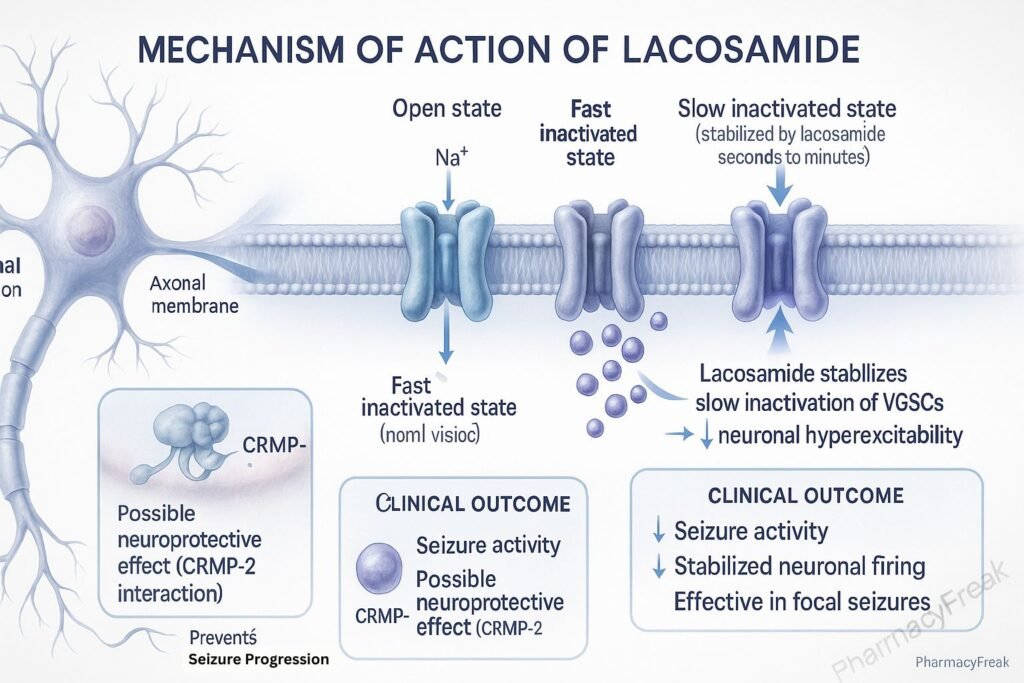
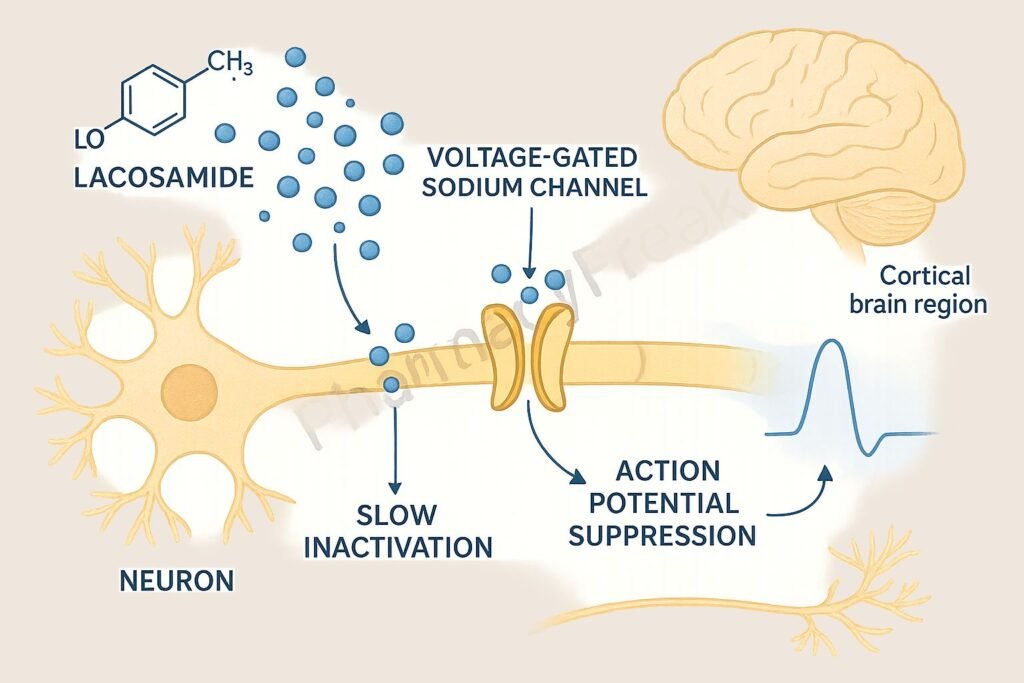
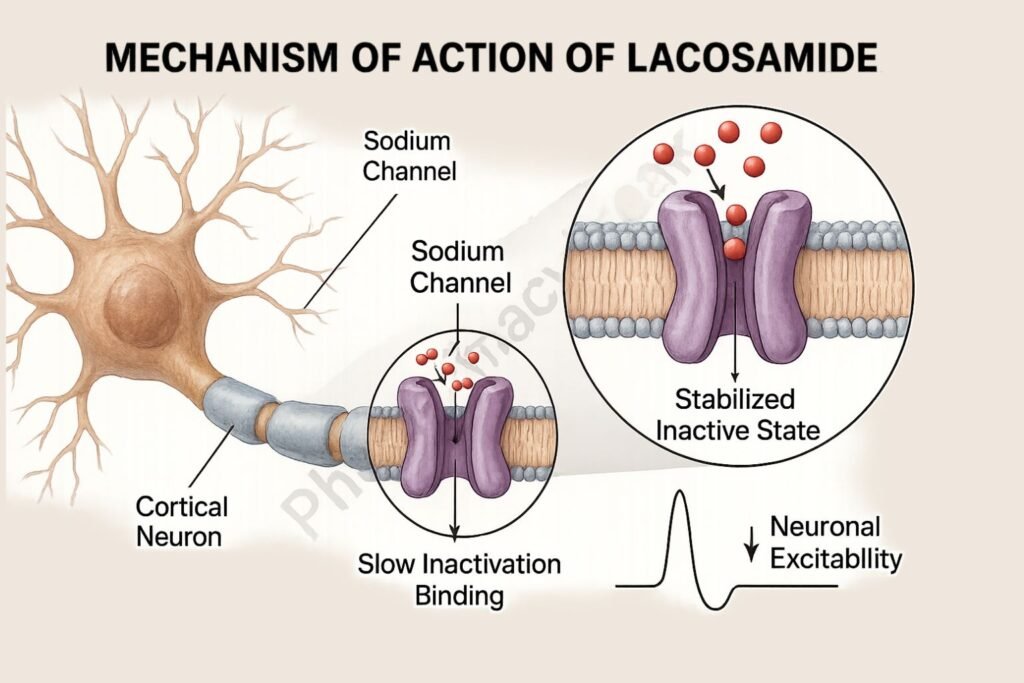
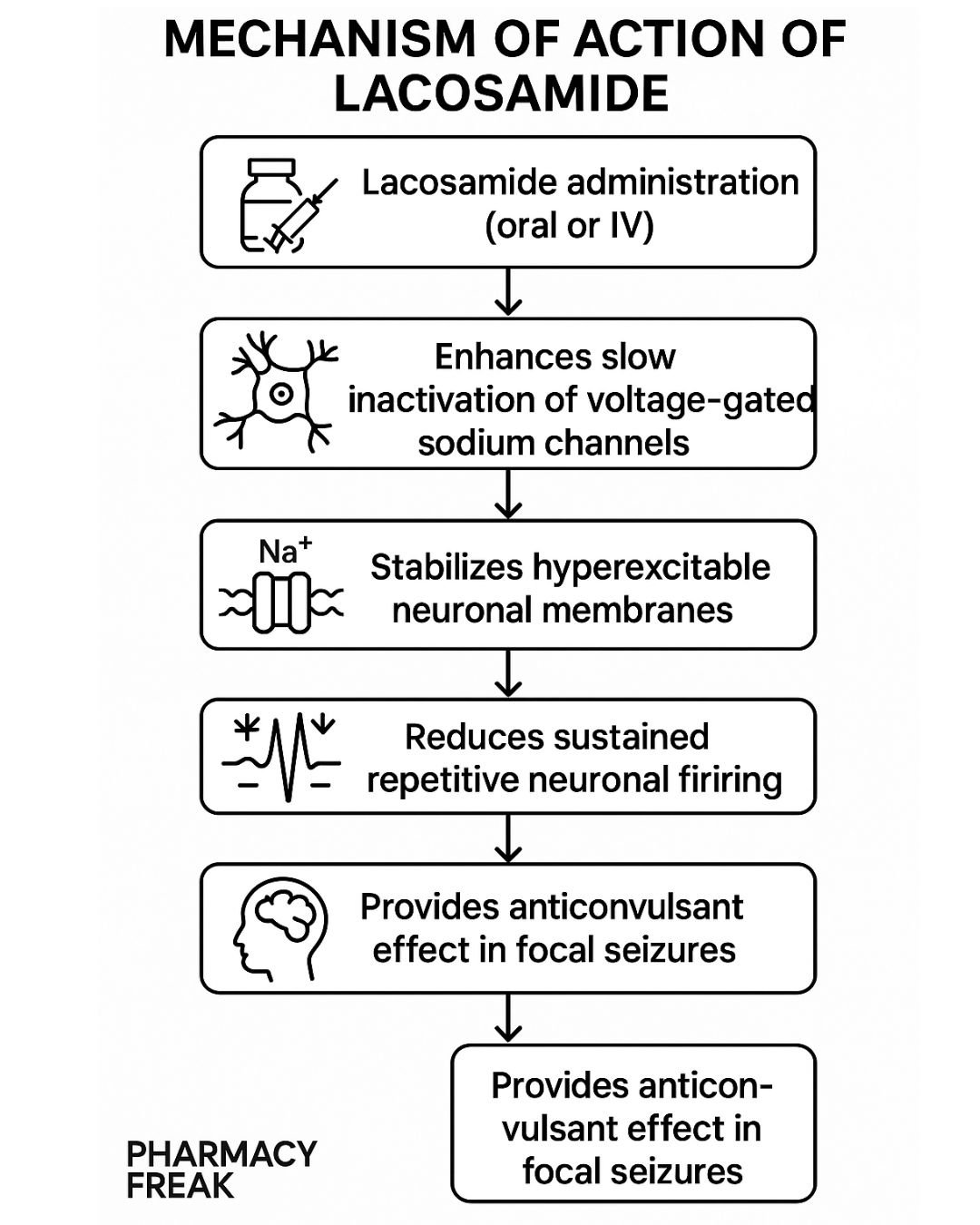
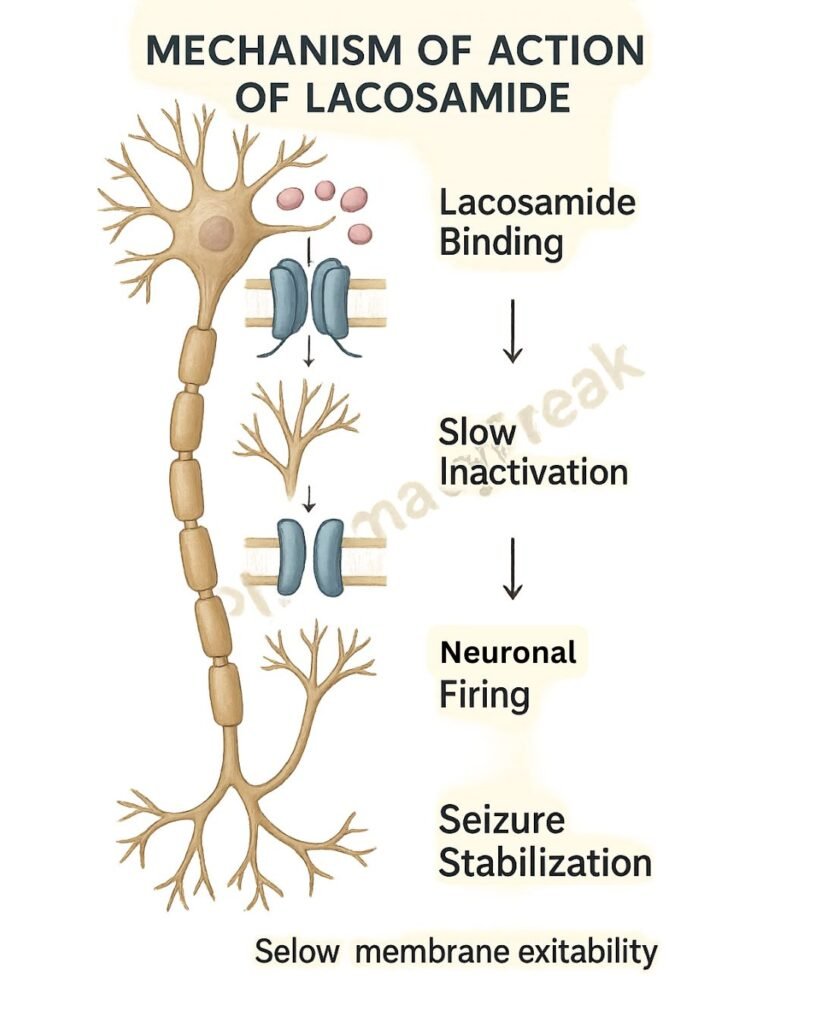
1. Binding to Voltage-Gated Sodium Channels
Lacosamide interacts with neuronal voltage-gated sodium channels.
2. Enhancement of Slow Inactivation
It promotes the slow inactivation state of sodium channels without affecting fast inactivation.
3. Stabilization of Hyperexcitable Neurons
This mechanism reduces the pathological hyperexcitability of neurons during sustained repetitive firing.
4. Inhibition of Seizure Propagation
The stabilization leads to diminished neuronal excitability, reducing the likelihood of seizure generation and spread.
5. Additional Mechanisms
Some evidence suggests lacosamide may modulate CRMP-2 (collapsin response mediator protein-2), contributing to neuroprotective effects, though this remains under investigation.
Pharmacokinetics
- Route of Administration: Oral and intravenous
- Bioavailability: ~100% (oral)
- Time to Peak Plasma: 1–4 hours (oral)
- Half-Life: ~13 hours
- Protein Binding: <15%
- Metabolism: Hepatic (CYP2C19 involvement, minor)
- Excretion: Primarily renal (~95% as unchanged drug and metabolites)
Clinical Uses
- Partial-onset seizures (monotherapy or adjunctive therapy)
- Adjunctive treatment of primary generalized tonic-clonic seizures
- Off-label: Neuropathic pain (under evaluation)
Adverse Effects
- Dizziness
- Headache
- Nausea and vomiting
- Diplopia
- Fatigue
- Cardiac conduction abnormalities (PR interval prolongation)
- Contraindications: Known hypersensitivity, caution in cardiac conduction disorders
Comparative Analysis
| Parameter | Lacosamide | Carbamazepine |
|---|---|---|
| Sodium Channel Effect | Enhances slow inactivation | Enhances fast inactivation |
| Drug Interactions | Minimal | Many (CYP inducer) |
| Dosing Frequency | Twice daily | Twice daily |
| Half-Life | ~13 hours | ~12 hours |
| Use in Elderly | Well-tolerated | More side effects |
Multiple Choice Questions (MCQs)
1. What is the primary mechanism of lacosamide?
a) Enhances GABA activity
b) Blocks calcium channels
c) Enhances slow inactivation of sodium channels
d) NMDA receptor antagonist
Answer: c) Enhances slow inactivation of sodium channels
2. Lacosamide is indicated for:
a) Absence seizures
b) Tonic-clonic seizures only
c) Partial-onset seizures
d) Status epilepticus
Answer: c) Partial-onset seizures
3. Which of the following is a common side effect of lacosamide?
a) Constipation
b) Dizziness
c) Alopecia
d) Agranulocytosis
Answer: b) Dizziness
4. Lacosamide should be used with caution in patients with:
a) Liver cirrhosis
b) Cardiac conduction abnormalities
c) Renal stones
d) Hypertension
Answer: b) Cardiac conduction abnormalities
5. What is the route of elimination for lacosamide?
a) Hepatic
b) Pulmonary
c) Renal
d) Biliary
Answer: c) Renal
FAQs
Q1. Is lacosamide used as monotherapy?
Yes, it is approved for monotherapy in partial-onset seizures.
Q2. Can lacosamide cause cardiac issues?
Yes, PR interval prolongation has been observed. ECG monitoring may be necessary in high-risk patients.
Q3. How is lacosamide administered?
It can be given orally or intravenously, making it suitable for acute and chronic settings.
Q4. Does lacosamide interact with many drugs?
No, it has minimal drug-drug interactions.
Q5. Is it safe in renal impairment?
Dose adjustment is recommended in moderate to severe renal impairment.
References
- Goodman & Gilman’s The Pharmacological Basis of Therapeutics, 12th Edition
- KD Tripathi, Essentials of Medical Pharmacology, 7th Edition
- FDA Prescribing Information for Lacosamide
- Clinical guidelines for seizure management
Related Internal Links

I am pursuing MBA in pharmaceutical management from NIPER Hyderabad with a strong academic record and proven success in national-level pharmacy entrance exams. I secured AIR 61 in NIPER 2024 (MS/M.Pharm) and AIR 27 in NIPER MBA, along with AIR 147 in GPAT 2024 and AIR 907 in GPAT 2023. I also achieved AIR 6 in AIIMS CRE-2025 for Drug Store Keeper and was selected as a Pharmacist (AIR 61) for ESIC. Additionally, I was the Runner-Up in Round 2 of the EY Case Study Competition.
At PharmacyFreak.com, I aim to guide future pharmacists through expert content, exam strategies, and insightful resources based on real experience and academic excellence.
Mail- harsh@pharmacyfreak.com