Table of Contents
Introduction
Codeine is a naturally occurring opioid analgesic and antitussive agent derived from opium. It is classified as a weak opioid and is commonly used for mild to moderate pain, cough suppression, and as an antidiarrheal in combination products. Codeine is a prodrug whose clinical effects largely depend on its metabolic conversion to morphine. Its pharmacology and pharmacogenetic variability make it a high-yield topic for pharmacology, anesthesia, and clinical medicine examinations.
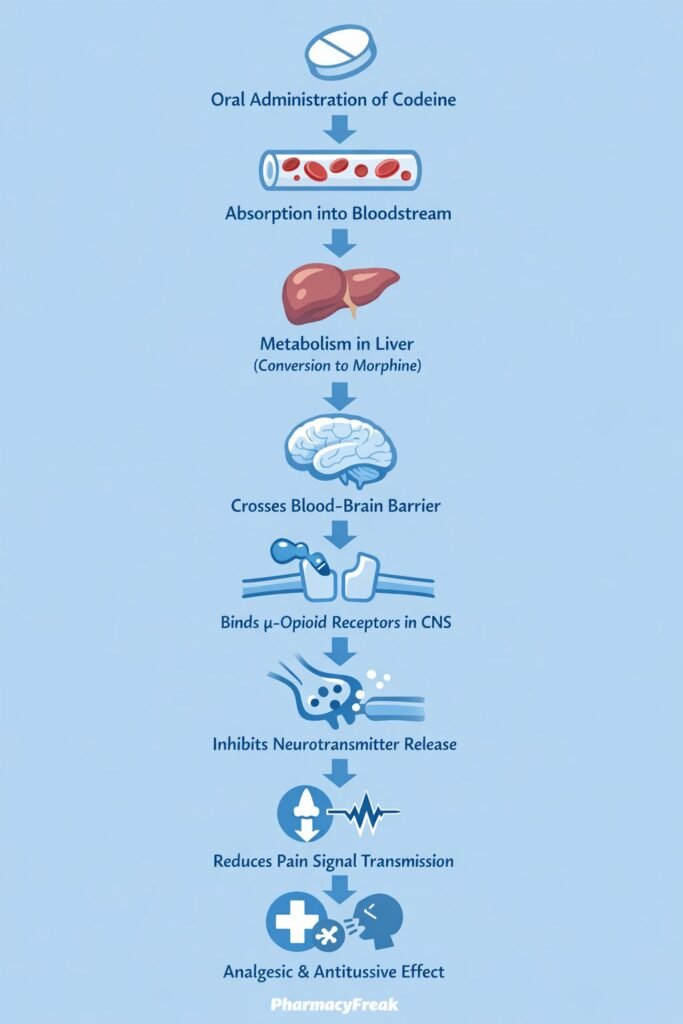
Mechanism of Action (Step-wise)
Codeine produces its effects primarily through opioid receptor activation, mainly after metabolic conversion to morphine.
Step-wise mechanism:
- Oral Administration and Absorption
Codeine is administered orally and absorbed from the gastrointestinal tract. - Hepatic Metabolism to Morphine
In the liver, codeine is O-demethylated by CYP2D6 to morphine, its active metabolite. - μ-Opioid Receptor Activation
Morphine (and to a lesser extent codeine itself) binds to μ-opioid receptors in the central nervous system. - G-Protein–Coupled Receptor Signaling
μ-opioid receptors are Gi-protein–coupled receptors that inhibit adenylate cyclase. - Reduced Neurotransmitter Release
This leads to:- Decreased cAMP production
- Closure of voltage-gated calcium channels
- Opening of potassium channels
- Neuronal Hyperpolarization
Reduced neurotransmitter release (e.g., substance P) decreases pain signal transmission in the spinal cord. - Central Effects
- Analgesia (pain relief)
- Antitussive action via suppression of the medullary cough center
- Sedation
Pharmacokinetics
- Absorption: Well absorbed orally
- Bioavailability: Approximately 60%
- Distribution: Widely distributed; crosses blood–brain barrier and placenta
- Metabolism: Hepatic metabolism via:
- CYP2D6 → morphine (active)
- CYP3A4 → norcodeine (inactive)
- Elimination: Renal excretion
- Half-life: Approximately 3 hours
Genetic polymorphisms of CYP2D6 significantly affect efficacy and toxicity.
Clinical Uses
Codeine is used for the following indications:
- Mild to moderate pain (often in combination with paracetamol or NSAIDs)
- Antitussive therapy for dry cough
- Symptomatic treatment of diarrhea (rare use)
- Postoperative and dental pain (limited use)
Because of safety concerns, its use is restricted in children and certain populations.
Adverse Effects
Common and serious adverse effects include:
- Central nervous system:
- Sedation
- Dizziness
- Gastrointestinal:
- Constipation
- Nausea
- Vomiting
- Respiratory:
- Respiratory depression (dose-dependent)
- Others:
- Miosis
- Pruritus
- Dependence and tolerance
Special concern:
Ultra-rapid CYP2D6 metabolizers may develop morphine toxicity even at standard doses.
Comparative Analysis (must include a table + explanation)
Comparison of Opioid Analgesics
| Feature | Codeine | Morphine | Tramadol |
|---|---|---|---|
| Analgesic potency | Low | High | Moderate |
| Prodrug | Yes | No | Partial |
| μ-receptor affinity | Weak | Strong | Weak |
| CYP2D6 dependence | High | None | Moderate |
| Abuse potential | Moderate | High | Moderate |
Explanation:
Codeine is less potent than morphine and requires metabolic activation, leading to variable clinical responses. Tramadol also depends on CYP2D6 but has additional monoaminergic effects. Morphine provides predictable analgesia but carries higher abuse and respiratory depression risk.
MCQs (10–15)
- Codeine primarily exerts its analgesic effect after conversion to:
a) Norcodeine
b) Morphine
c) Hydromorphone
d) Naloxone
Answer: b) Morphine
- Codeine activates which receptor to produce analgesia?
a) κ-opioid
b) δ-opioid
c) μ-opioid
d) NMDA
Answer: c) μ-opioid
- The enzyme responsible for converting codeine to morphine is:
a) CYP3A4
b) CYP2C9
c) CYP2D6
d) CYP1A2
Answer: c) CYP2D6
- Codeine decreases pain transmission by:
a) Increasing cAMP
b) Opening sodium channels
c) Inhibiting calcium channels
d) Activating NMDA receptors
Answer: c) Inhibiting calcium channels
- Which population is at highest risk of codeine toxicity?
a) Poor metabolizers
b) Ultra-rapid CYP2D6 metabolizers
c) Elderly patients
d) Smokers
Answer: b) Ultra-rapid CYP2D6 metabolizers
- Codeine is classified as:
a) Strong opioid
b) Weak opioid
c) Non-opioid analgesic
d) NSAID
Answer: b) Weak opioid
- Codeine suppresses cough by acting on:
a) Bronchial smooth muscle
b) Peripheral receptors
c) Medullary cough center
d) Vagal afferents
Answer: c) Medullary cough center
- A common gastrointestinal adverse effect of codeine is:
a) Diarrhea
b) Constipation
c) GI bleeding
d) Pancreatitis
Answer: b) Constipation
- Codeine causes respiratory depression by:
a) Stimulating respiratory center
b) Increasing CO₂ sensitivity
c) Suppressing brainstem respiratory drive
d) Blocking acetylcholine
Answer: c) Suppressing brainstem respiratory drive
- Codeine should be avoided in children because of:
a) Poor absorption
b) Risk of morphine toxicity
c) Ineffectiveness
d) Hepatotoxicity
Answer: b) Risk of morphine toxicity
FAQs (minimum 5)
- Is codeine a prodrug?
Yes, its analgesic effect mainly depends on conversion to morphine. - Why does codeine show variable analgesic effects?
Due to genetic polymorphisms in CYP2D6. - Can codeine cause respiratory depression?
Yes, especially in high doses or ultra-rapid metabolizers. - Why is codeine restricted in pediatric use?
Because of unpredictable conversion to morphine and risk of fatal respiratory depression. - Does codeine cause addiction?
Yes, prolonged use can lead to dependence and tolerance. - Is codeine effective as a cough suppressant?
Yes, it suppresses the medullary cough center.
References
- Goodman & Gilman’s The Pharmacological Basis of Therapeutics
https://accessmedicine.mhmedical.com - Katzung BG. Basic and Clinical Pharmacology
https://accessmedicine.mhmedical.com - Tripathi KD. Essentials of Medical Pharmacology
https://www.jaypeebrothers.com - Harrison’s Principles of Internal Medicine
https://accessmedicine.mhmedical.com

I am pursuing MBA in pharmaceutical management from NIPER Hyderabad with a strong academic record and proven success in national-level pharmacy entrance exams. I secured AIR 61 in NIPER 2024 (MS/M.Pharm) and AIR 27 in NIPER MBA, along with AIR 147 in GPAT 2024 and AIR 907 in GPAT 2023. I also achieved AIR 6 in AIIMS CRE-2025 for Drug Store Keeper and was selected as a Pharmacist (AIR 61) for ESIC. Additionally, I was the Runner-Up in Round 2 of the EY Case Study Competition.
At PharmacyFreak.com, I aim to guide future pharmacists through expert content, exam strategies, and insightful resources based on real experience and academic excellence.
Mail- harsh@pharmacyfreak.com
