Table of Contents
Introduction
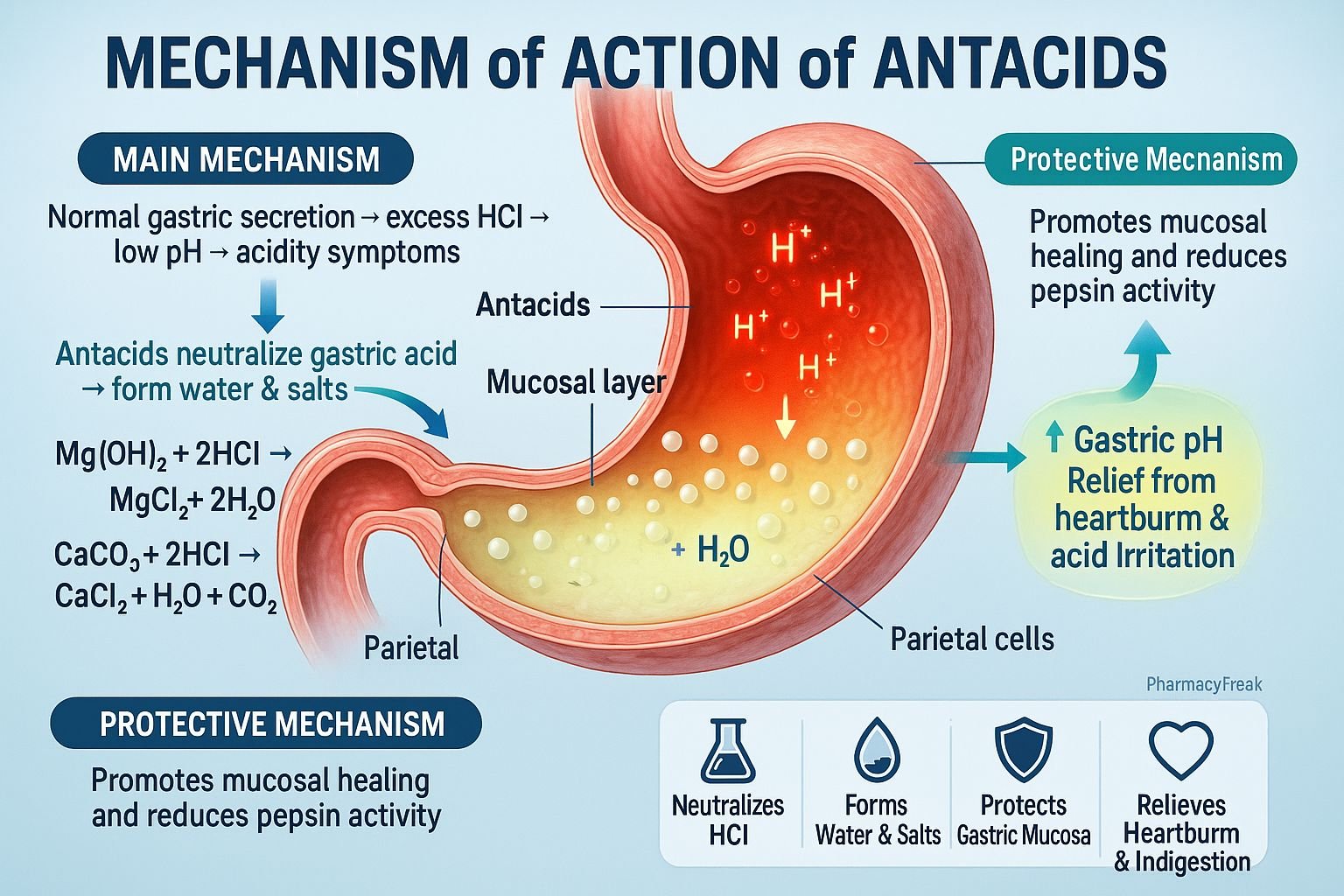
Antacids are weakly basic compounds that neutralize excess gastric acid in the stomach, providing rapid relief from heartburn, acid reflux, gastritis, and peptic ulcer disease. Mechanism of Action of Antacids involves a direct chemical reaction with hydrochloric acid (HCl) in gastric juice to form salt and water, thereby increasing gastric pH and reducing acidity. They act locally, not systemically, and are among the oldest and most widely used medications for acid-related disorders.
Mechanism of Action (Step-wise)
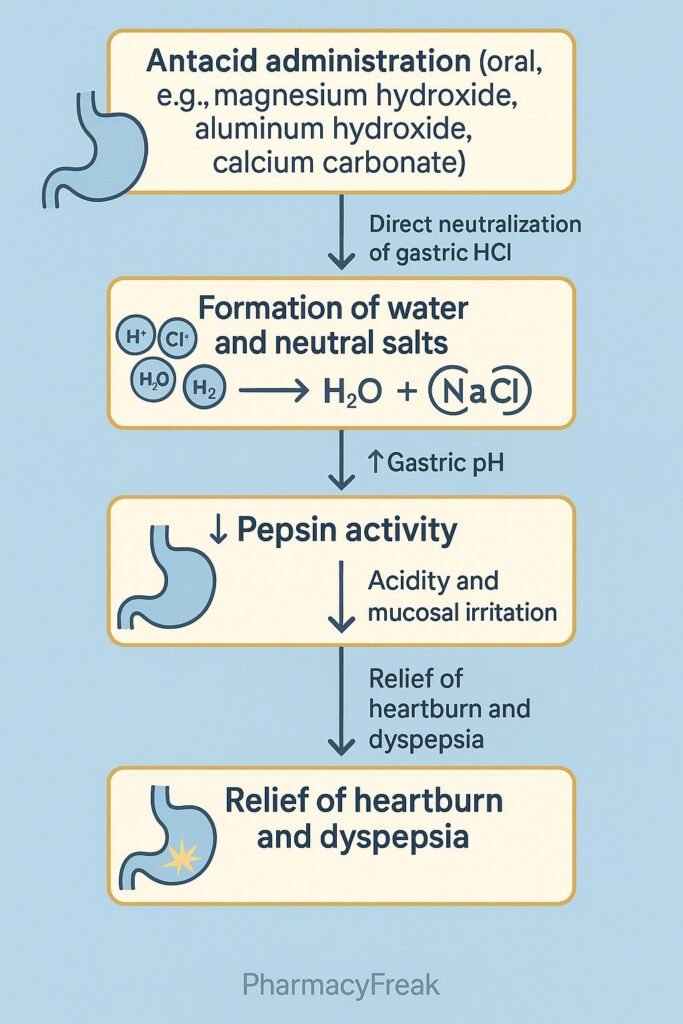
- Neutralization of Gastric Acid
- Antacids are weak bases that react with hydrochloric acid (HCl) in the stomach.
- The chemical reaction neutralizes acid, forming salt (NaCl, MgCl₂, CaCl₂, or AlCl₃) and water (H₂O).
- Example:
- 2HCl+CaCO3→CaCl2+CO2+H2O2HCl + CaCO_3 → CaCl_2 + CO_2 + H_2O2HCl+CaCO3→CaCl2+CO2+H2O
- Increase in Gastric pH
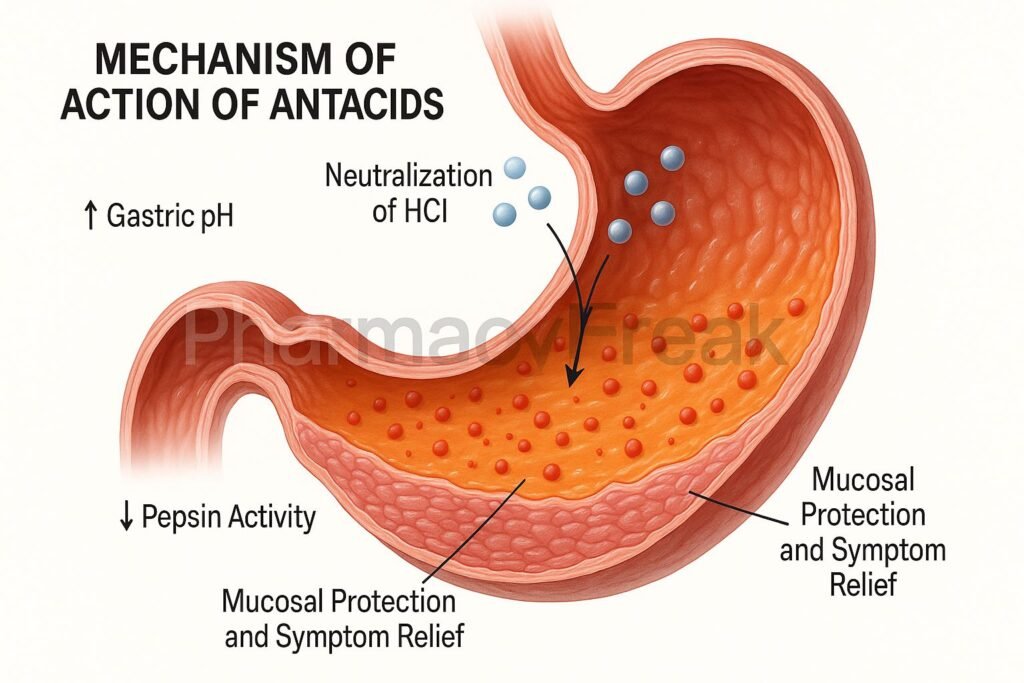
- Neutralization elevates the gastric pH above 4, reducing the activity of pepsin, an enzyme responsible for mucosal damage.
- Pepsin becomes inactive at higher pH levels, protecting the gastric mucosa from further erosion.
- Relief of Pain and Discomfort
- The rise in gastric pH rapidly alleviates burning epigastric pain and acid reflux symptoms.
- Mucosal Protection
- Some antacids (especially aluminum hydroxide) stimulate prostaglandin production, enhancing mucosal defense and healing.
- Secondary Effects
- Carbonate-containing antacids release carbon dioxide (CO₂), causing belching.
- Magnesium-containing antacids promote osmotic laxative effects, while aluminum compounds may cause constipation.
- Overall Effect
- HCl neutralization → pH elevation → pepsin inhibition → mucosal protection → symptomatic relief.
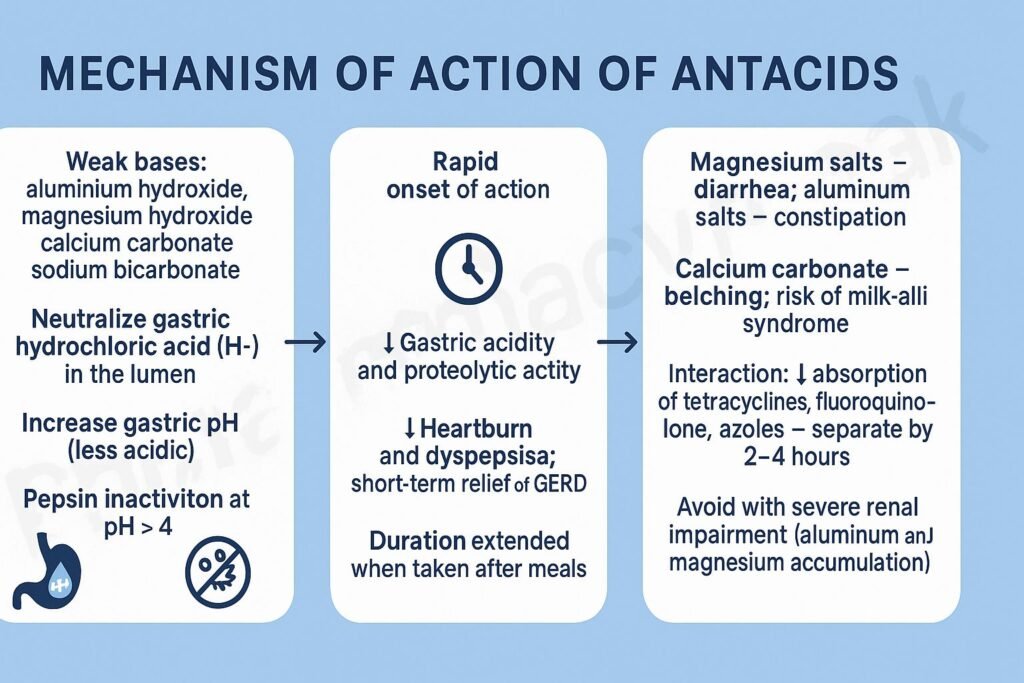
Pharmacokinetics
- Onset: Rapid (within 5–15 minutes).
- Duration: 1–2 hours; shorter if taken on an empty stomach.
- Absorption: Most act locally; minimal systemic absorption.
- Excretion: Insoluble salts excreted in feces; minimal renal elimination.
Clinical Uses
- Peptic ulcer disease (symptomatic relief)
- Gastroesophageal reflux disease (GERD)
- Dyspepsia and heartburn
- Gastritis and hyperacidity
- Adjunct therapy with H₂ blockers or proton pump inhibitors (PPIs)
Adverse Effects
- Aluminum hydroxide: Constipation, hypophosphatemia.
- Magnesium hydroxide: Diarrhea, hypermagnesemia (in renal failure).
- Calcium carbonate: Hypercalcemia, rebound acid secretion (“acid rebound”).
- Sodium bicarbonate: Metabolic alkalosis, gas/bloating.
- General: Belching, altered bowel habits, electrolyte imbalance.
Comparative Analysis
| Feature | Aluminum Hydroxide | Magnesium Hydroxide | Calcium Carbonate | Sodium Bicarbonate |
|---|---|---|---|---|
| Onset of action | Moderate | Rapid | Moderate | Very rapid |
| Duration of effect | Long | Short | Long | Short |
| Side effect | Constipation | Diarrhea | Hypercalcemia, belching | Metabolic alkalosis |
| Combination therapy | Often combined with Mg(OH)₂ | Often combined with Al(OH)₃ | Alone or with simethicone | Rarely used alone |
MCQs
1. The primary mechanism of action of antacids is:
a) Inhibition of acid secretion
b) Neutralization of gastric acid
c) Blocking H₂ receptors
d) Inhibition of proton pumps
Answer: b) Neutralization of gastric acid
2. Which of the following antacids causes constipation?
a) Magnesium hydroxide
b) Aluminum hydroxide
c) Calcium carbonate
d) Sodium bicarbonate
Answer: b) Aluminum hydroxide
3. Antacids relieve pain by:
a) Inhibiting pepsin activity
b) Blocking gastrin receptors
c) Reducing mucus secretion
d) Stimulating HCl production
Answer: a) Inhibiting pepsin activity
4. Which antacid can cause diarrhea?
a) Calcium carbonate
b) Aluminum hydroxide
c) Magnesium hydroxide
d) Sodium bicarbonate
Answer: c) Magnesium hydroxide
5. CO₂ formation and belching occur with:
a) Aluminum hydroxide
b) Calcium carbonate
c) Magnesium trisilicate
d) None of the above
Answer: b) Calcium carbonate
6. Which antacid can cause metabolic alkalosis?
a) Sodium bicarbonate
b) Aluminum hydroxide
c) Magnesium hydroxide
d) Calcium carbonate
Answer: a) Sodium bicarbonate
7. Antacids act:
a) Locally in the stomach
b) Systemically via circulation
c) On parietal cells
d) On vagal nerve endings
Answer: a) Locally in the stomach
8. Which enzyme’s activity is inhibited by increased gastric pH?
a) Pepsin
b) Trypsin
c) Amylase
d) Lipase
Answer: a) Pepsin
9. The combination of aluminum and magnesium antacids helps to:
a) Increase acid secretion
b) Balance constipation and diarrhea effects
c) Reduce absorption
d) Increase CO₂ formation
Answer: b) Balance constipation and diarrhea effects
10. Sodium bicarbonate should be avoided in:
a) Hypertension and heart failure
b) Peptic ulcer
c) GERD
d) Acid reflux
Answer: a) Hypertension and heart failure
FAQs
Q1. How do antacids work?
They neutralize excess gastric acid, increasing pH and reducing acidity.
Q2. When should antacids be taken?
Preferably 1 hour after meals and at bedtime for maximum effect.
Q3. Can antacids be taken with other medications?
They can interfere with drug absorption (e.g., tetracyclines, fluoroquinolones); spacing doses by 2 hours is recommended.
Q4. What’s the difference between antacids and PPIs?
Antacids provide immediate symptomatic relief, while PPIs suppress acid production long-term.
Q5. Are antacids safe in pregnancy?
Yes, most are safe in pregnancy when used in recommended doses.
Q6. Can overuse of antacids cause problems?
Yes, chronic use can lead to electrolyte imbalance and alkalosis.
References
- Goodman & Gilman’s The Pharmacological Basis of Therapeutics
- Katzung’s Basic and Clinical Pharmacology
- Harrison’s Principles of Internal Medicine
- FDA OTC Drug Monograph for Antacids
Related Internal Links

I am pursuing MBA in pharmaceutical management from NIPER Hyderabad with a strong academic record and proven success in national-level pharmacy entrance exams. I secured AIR 61 in NIPER 2024 (MS/M.Pharm) and AIR 27 in NIPER MBA, along with AIR 147 in GPAT 2024 and AIR 907 in GPAT 2023. I also achieved AIR 6 in AIIMS CRE-2025 for Drug Store Keeper and was selected as a Pharmacist (AIR 61) for ESIC. Additionally, I was the Runner-Up in Round 2 of the EY Case Study Competition.
At PharmacyFreak.com, I aim to guide future pharmacists through expert content, exam strategies, and insightful resources based on real experience and academic excellence.
Mail- harsh@pharmacyfreak.com