Table of Contents
Introduction
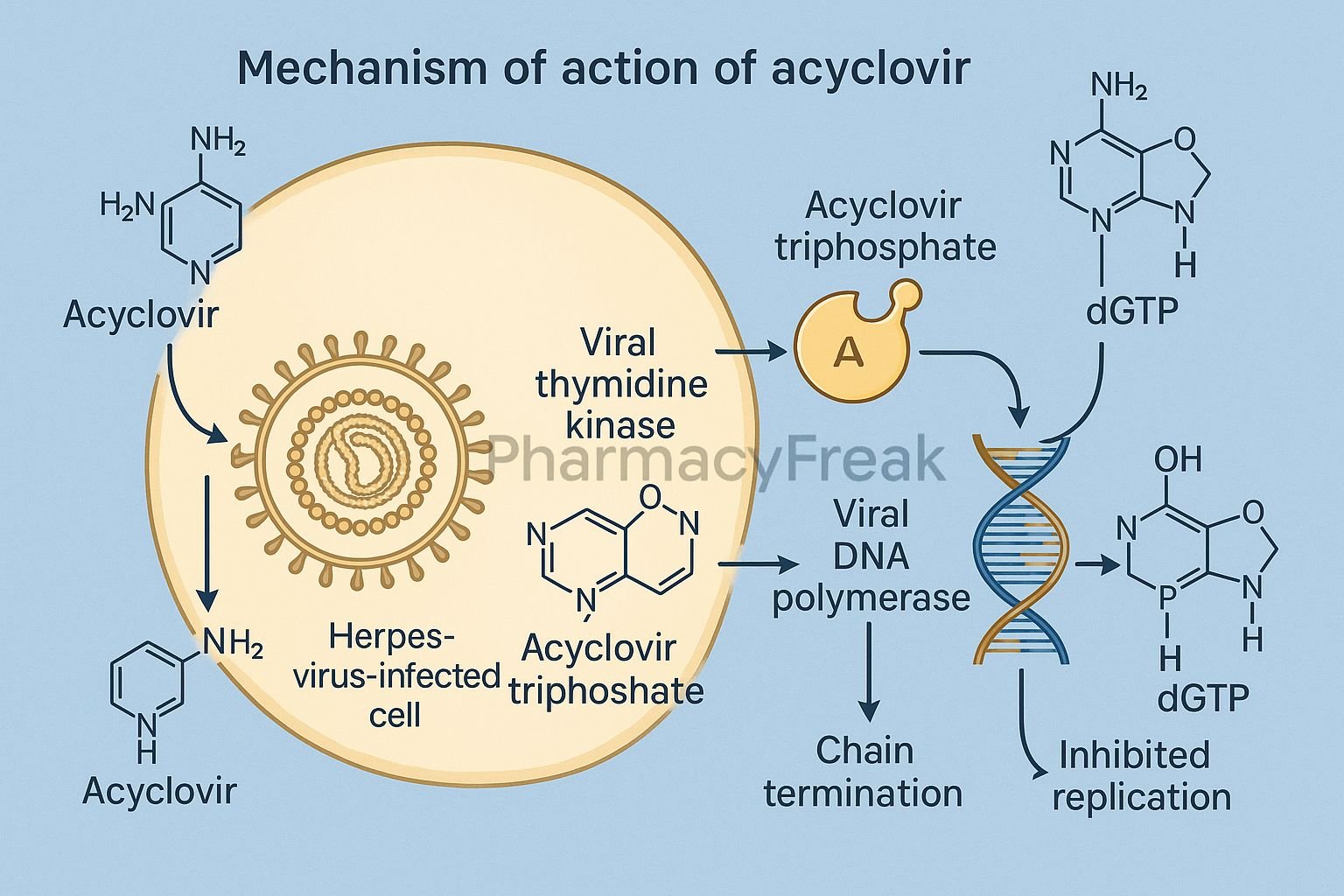
Acyclovir is a guanine nucleoside analogue antiviral drug primarily used to treat infections caused by herpes simplex virus (HSV-1 and HSV-2) and varicella-zoster virus (VZV). Mechanism of Action of Acyclovir involves selective inhibition of viral DNA synthesis, resulting in the termination of viral replication. It is widely prescribed for herpes labialis, genital herpes, herpes zoster, and varicella infections. Due to its selective activation in virus-infected cells, acyclovir has excellent antiviral efficacy with minimal toxicity to host cells.
Mechanism of Action (Step-wise)
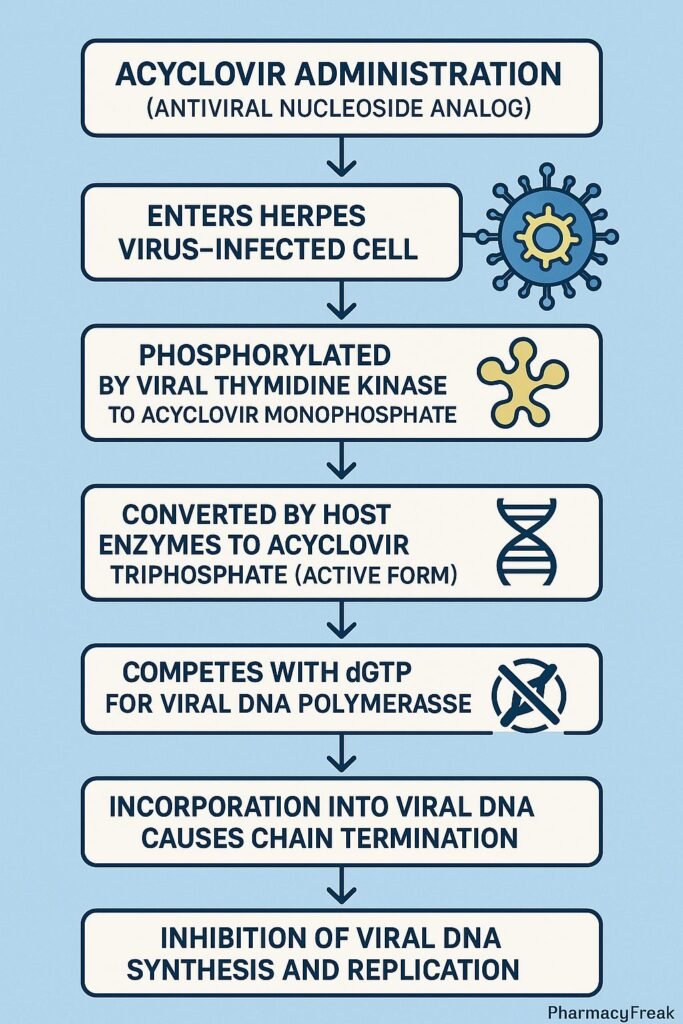
- Viral Entry and Activation
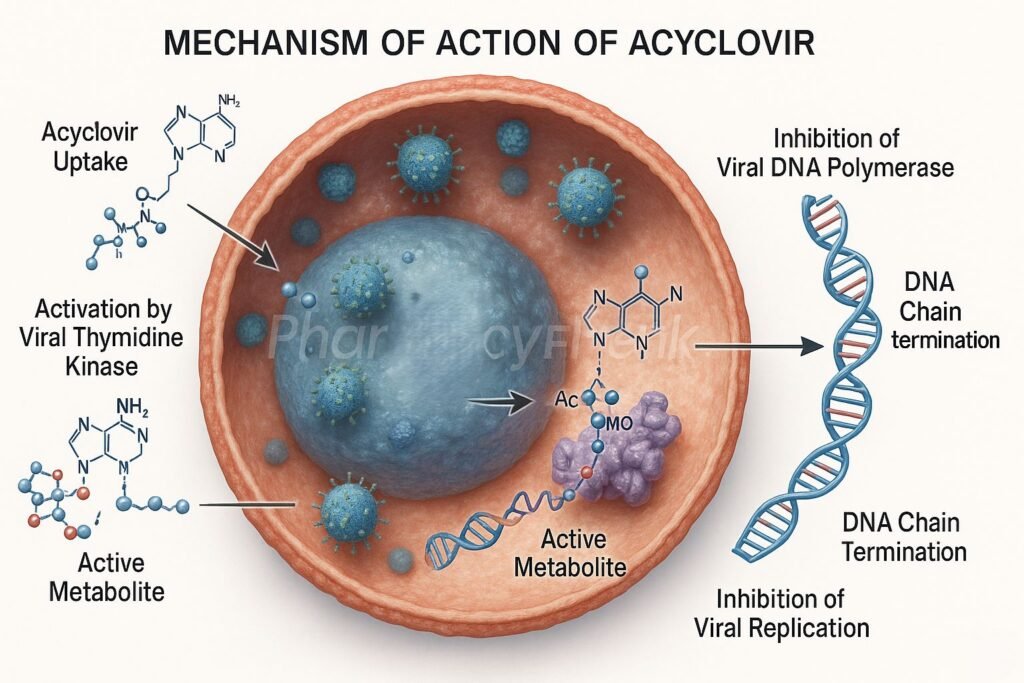
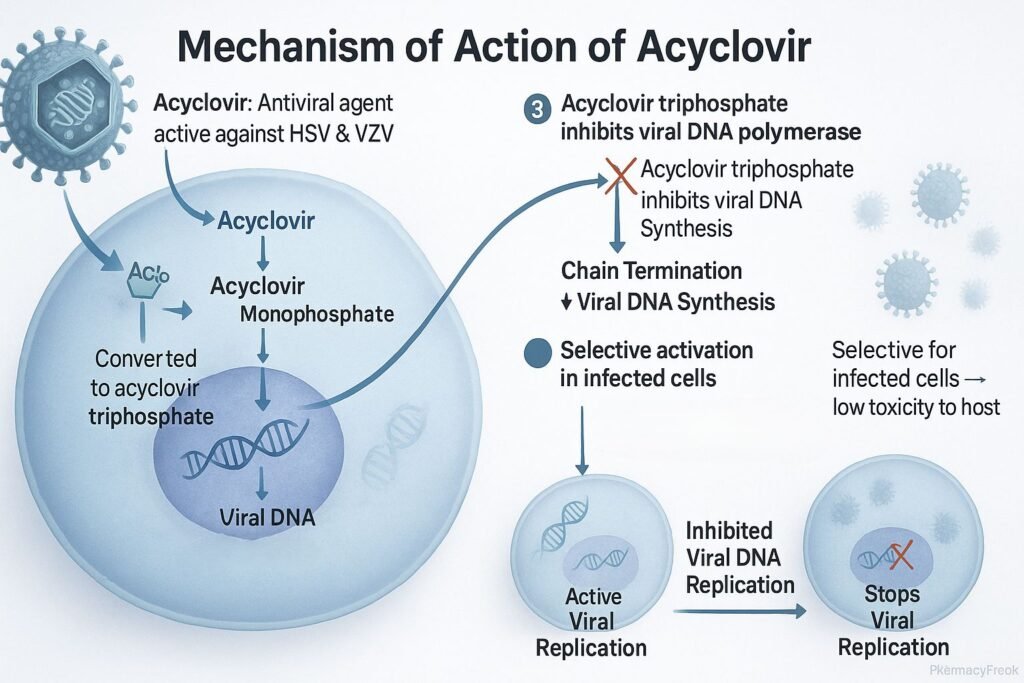
- Acyclovir enters both infected and uninfected host cells.
- In virus-infected cells, the viral enzyme thymidine kinase (TK) phosphorylates acyclovir to acyclovir monophosphate.
- Conversion to Active Triphosphate Form
- Host cell kinases further phosphorylate it to acyclovir triphosphate (ACV-TP), the pharmacologically active form.
- Inhibition of Viral DNA Polymerase
- ACV-TP competitively inhibits viral DNA polymerase, preventing incorporation of natural deoxyguanosine triphosphate (dGTP).
- This blocks the elongation of the viral DNA chain.
- Chain Termination
- Because acyclovir lacks the 3’-hydroxyl group required for DNA chain elongation, insertion of ACV-TP results in premature termination of viral DNA synthesis.
- Selective Toxicity
- Uninfected cells lack viral thymidine kinase, so they convert acyclovir inefficiently, minimizing host cell toxicity.
- Overall Effect
- Inhibition of viral replication and reduction in viral load.
- Limits spread and duration of herpes infections.
Pharmacokinetics
- Absorption: Oral bioavailability ~15–30%; improved with prodrug valacyclovir.
- Distribution: Widely distributed, including cerebrospinal fluid (CSF).
- Metabolism: Minimal hepatic metabolism.
- Excretion: Primarily unchanged in urine via glomerular filtration and tubular secretion.
- Half-life: 2–3 hours (may extend to 20 hours in renal impairment).
Clinical Uses
- Herpes simplex virus infections:
- HSV-1 (oral herpes) and HSV-2 (genital herpes).
- Varicella-zoster virus (VZV):
- Shingles (herpes zoster) and chickenpox.
- Herpes simplex encephalitis.
- Prophylaxis in immunocompromised patients.
- Ocular herpes infections (as ophthalmic ointment).
Adverse Effects
- Common: Nausea, headache, diarrhea, and fatigue.
- Less common: Rash, dizziness, mild gastrointestinal upset.
- Serious: Nephrotoxicity (especially with IV use and dehydration), neurotoxicity (tremors, confusion), and crystalluria.
- Contraindications: Caution in renal impairment; adequate hydration required during IV therapy.
Comparative Analysis
| Feature | Acyclovir | Valacyclovir (Prodrug) | Famciclovir |
|---|---|---|---|
| Bioavailability | 15–30% | 55–70% | 75% |
| Active form | Acyclovir triphosphate | Acyclovir (after conversion) | Penciclovir triphosphate |
| Activation enzyme | Viral thymidine kinase | Viral thymidine kinase | Viral thymidine kinase |
| Dosing frequency | Frequent (5×/day oral) | Less frequent (2–3×/day) | 2–3×/day |
| Clinical efficacy | High for HSV, VZV | Similar, with better compliance | Similar efficacy |
MCQs
1. Acyclovir acts primarily by inhibiting:
a) Reverse transcriptase
b) Viral DNA polymerase
c) RNA polymerase
d) Integrase enzyme
Answer: b) Viral DNA polymerase
2. The active form of acyclovir is:
a) Acyclovir monophosphate
b) Acyclovir diphosphate
c) Acyclovir triphosphate
d) Acyclovir base
Answer: c) Acyclovir triphosphate
3. The first phosphorylation of acyclovir is catalyzed by:
a) Host cell kinase
b) Viral thymidine kinase
c) Adenylate cyclase
d) Guanylate cyclase
Answer: b) Viral thymidine kinase
4. The absence of which group leads to chain termination in DNA synthesis?
a) 5′-phosphate
b) 3′-hydroxyl
c) Amino group
d) Sulfhydryl group
Answer: b) 3′-hydroxyl
5. Which of the following enhances acyclovir oral bioavailability?
a) Co-administration with milk
b) Conversion to valacyclovir
c) High-fat meals
d) Acidic urine
Answer: b) Conversion to valacyclovir
6. The major route of excretion of acyclovir is:
a) Bile
b) Sweat
c) Renal excretion
d) Pulmonary exhalation
Answer: c) Renal excretion
7. Which of the following infections is NOT treated with acyclovir?
a) Herpes simplex
b) Cytomegalovirus
c) Varicella-zoster
d) Herpes encephalitis
Answer: b) Cytomegalovirus
8. Nephrotoxicity with IV acyclovir occurs due to:
a) Hepatic enzyme inhibition
b) Crystal precipitation in renal tubules
c) Immune complex formation
d) Decreased urine output
Answer: b) Crystal precipitation in renal tubules
9. The prodrug of acyclovir is:
a) Valacyclovir
b) Famciclovir
c) Penciclovir
d) Ganciclovir
Answer: a) Valacyclovir
10. Acyclovir resistance in HSV usually arises due to:
a) Mutation in viral thymidine kinase
b) Mutation in human DNA polymerase
c) Overexpression of host kinase
d) Efflux pump activation
Answer: a) Mutation in viral thymidine kinase
FAQs
Q1. Is acyclovir effective against all herpes viruses?
Primarily against HSV-1, HSV-2, and VZV; less effective against CMV and EBV.
Q2. Can acyclovir cure herpes infection?
No, it controls replication and symptoms but does not eradicate latent virus.
Q3. How should acyclovir be taken for oral herpes?
Start within 24–48 hours of symptom onset for best results.
Q4. What is the difference between acyclovir and valacyclovir?
Valacyclovir is the L-valyl ester prodrug of acyclovir with higher bioavailability.
Q5. How can nephrotoxicity from acyclovir be prevented?
Adequate hydration and slow IV infusion reduce risk.
Q6. Is acyclovir safe during pregnancy?
Yes, category B — safe for use when benefits outweigh risks.
References
- Goodman & Gilman’s The Pharmacological Basis of Therapeutics
- Katzung’s Basic and Clinical Pharmacology
- Harrison’s Principles of Internal Medicine
- Clinical Virology and Antiviral Therapy Guidelines
Related Internal Links

I am pursuing MBA in pharmaceutical management from NIPER Hyderabad with a strong academic record and proven success in national-level pharmacy entrance exams. I secured AIR 61 in NIPER 2024 (MS/M.Pharm) and AIR 27 in NIPER MBA, along with AIR 147 in GPAT 2024 and AIR 907 in GPAT 2023. I also achieved AIR 6 in AIIMS CRE-2025 for Drug Store Keeper and was selected as a Pharmacist (AIR 61) for ESIC. Additionally, I was the Runner-Up in Round 2 of the EY Case Study Competition.
At PharmacyFreak.com, I aim to guide future pharmacists through expert content, exam strategies, and insightful resources based on real experience and academic excellence.
Mail- harsh@pharmacyfreak.com