Vaccines are one of the most effective public health tools developed to prevent infectious diseases. They work by stimulating the body’s immune system to recognize and combat pathogens. With the development of new biotechnology platforms, the scope and classification of vaccines have expanded significantly.
This blog from Pharmacy Freak explains the classification of vaccines, their uses, common choices in clinical practice, and recent developments relevant for academic and exam purposes.
Table of Contents
What is Vaccines
A vaccine is a biological preparation that provides active acquired immunity to a particular infectious disease. It typically contains an agent resembling a disease-causing microorganism and is often made from weakened or killed forms of the microbe, its toxins, or one of its surface proteins.
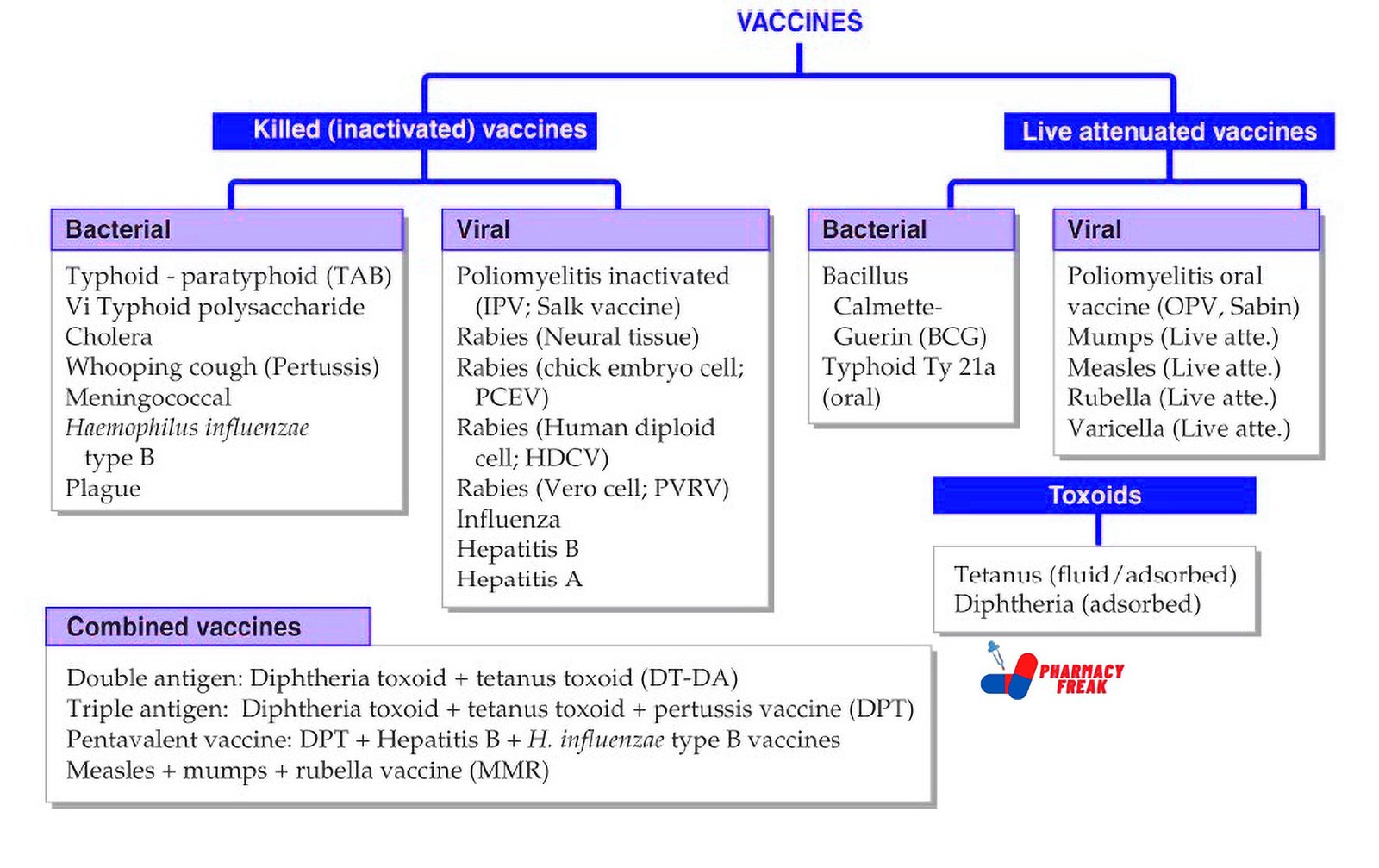
Classification of Vaccines (KD Tripathi)
- Killed (inactivated) vaccines –
Bacterial: Typhoid-paratyphoid (TAB), Vi Typhoid polysaccharide, Cholera, Whooping cough (Pertussis), Meningococcal, Haemophilus influenzae type B, Plague
Viral: Poliomyelitis inactivated (IPV; Salk vaccine), Rabies (Neural tissue), Rabies (chick embryo cell; PCEV), Rabies (Human diploid cell; HDCV), Rabies (Vero cell; PVRV), Influenza, Hepatitis B, Hepatitis A - Live attenuated vaccines –
Bacterial: Bacillus Calmette–Guérin (BCG), Typhoid Ty21a (oral)
Viral: Poliomyelitis oral vaccine (OPV; Sabin), Mumps (Live attenuated), Measles (Live attenuated), Rubella (Live attenuated), Varicella (Live attenuated) - Toxoids –
Tetanus (fluid/adsorbed), Diphtheria (adsorbed) - Combined vaccines –
Double antigen: Diphtheria toxoid + Tetanus toxoid (DT-DA)
Triple antigen: Diphtheria toxoid + Tetanus toxoid + Pertussis vaccine (DPT)
Pentavalent vaccine: DPT + Hepatitis B + H. influenzae type B vaccines
Measles + Mumps + Rubella vaccine (MMR)
Classification (General)
Vaccines are classified based on the nature of the antigen used and the method of preparation:
- Live Attenuated Vaccines
These contain weakened forms of the live pathogen that can replicate but do not cause disease in healthy individuals.
Examples: Measles, Mumps, Rubella (MMR), BCG, Oral Polio Vaccine (OPV), Varicella, Yellow Fever
Features: Strong and long-lasting immunity, often lifelong with a single dose
Caution: Contraindicated in immunocompromised individuals - Inactivated (Killed) Vaccines
These contain pathogens that have been killed by heat, radiation, or chemicals.
Examples: Inactivated Polio Vaccine (IPV), Hepatitis A, Rabies, Influenza (injectable)
Features: Safe in immunocompromised patients, but require booster doses - Subunit, Recombinant, Polysaccharide, and Conjugate Vaccines
These use specific pieces of the pathogen such as protein or sugar antigens.
Examples:
- Hepatitis B (recombinant surface antigen)
- HPV vaccine (recombinant virus-like particles)
- Pneumococcal conjugate vaccine (PCV)
- Meningococcal vaccine
- Haemophilus influenzae type b (Hib) conjugate vaccine
Features: Highly specific, fewer side effects, used in pediatric schedules
- Toxoid Vaccines
These contain inactivated toxins produced by the pathogen rather than the organism itself.
Examples: Tetanus toxoid, Diphtheria toxoid
Features: Require booster doses; generate immunity against the toxin, not the organism - mRNA Vaccines
These vaccines use messenger RNA to instruct cells to produce a harmless piece of the virus protein, triggering an immune response.
Examples: COVID-19 vaccines (Pfizer-BioNTech, Moderna)
Features: Rapid production, no live virus involved, stable and highly immunogenic - Viral Vector Vaccines
These use a modified virus (vector) to deliver genetic instructions for producing antigenic proteins.
Examples: Covishield, Johnson & Johnson COVID-19 vaccine
Features: Strong immune response; may be live or non-replicating vector types - DNA Vaccines (Investigational)
Contain genetically engineered DNA that induces cells to produce antigen.
Still under research; some veterinary applications exist
Uses
Vaccines are used in the prevention of:
- Childhood infectious diseases (e.g., DPT, MMR, Polio, Hib)
- Hepatitis A and B
- Human Papillomavirus (HPV) infection to prevent cervical cancer
- Seasonal Influenza and pandemic threats (e.g., COVID-19)
- Rabies post-exposure prophylaxis
- Travel-related diseases (e.g., Yellow fever, Typhoid)
Drug of Choice Highlights
- Routine childhood immunization – Pentavalent vaccine (DPT + Hib + Hep B)
- Tuberculosis prevention – BCG vaccine (Live attenuated)
- COVID-19 – mRNA vaccine (Pfizer-BioNTech) or viral vector vaccine (Covishield)
- Hepatitis B – Recombinant surface antigen vaccine
- Tetanus prophylaxis – Tetanus toxoid
- Typhoid – Typhoid conjugate vaccine (TCV)
Side Effects
- Live vaccines – Mild fever, rash, local reaction; may rarely revert to virulence
- Killed vaccines – Local pain, swelling, and fever
- Subunit and conjugate vaccines – Mild local reactions, generally well tolerated
- Toxoids – Mild fever, pain at injection site
- mRNA vaccines – Fatigue, fever, headache, rare anaphylaxis
- Viral vector vaccines – Fever, myalgia, very rare thrombosis with thrombocytopenia
Updated Clinical Pearls
- Live attenuated vaccines offer lifelong immunity with fewer doses but are contraindicated in immunocompromised and pregnant individuals.
- Inactivated vaccines are safer but usually require booster doses to maintain immunity.
- Conjugate vaccines improve immune response in young children where polysaccharide vaccines are less effective.
- mRNA vaccines have revolutionized vaccine technology by enabling faster development and strong protection.
- Combination vaccines like pentavalent reduce the number of injections and improve compliance.
- Storage and cold chain maintenance are critical, especially for mRNA and live vaccines.
References
- Tripathi KD. Essentials of Medical Pharmacology. 7th ed. New Delhi: Jaypee Brothers Medical Publishers; 2013. p. 141–146
- Gupta S, Garg A. Review of Pharmacology. 15th ed. New Delhi: Jaypee Brothers Medical Publishers; 2023. p. 308–312
- Brunton LL, Chabner BA, Knollmann BC, editors. Goodman and Gilman’s The Pharmacological Basis of Therapeutics. 12th ed. New York: McGraw-Hill Education; 2011. p. 1419–1427
Related Links

I am a Registered Pharmacist under the Pharmacy Act, 1948, and the founder of PharmacyFreak.com. I hold a Bachelor of Pharmacy degree from Rungta College of Pharmaceutical Science and Research. With a strong academic foundation and practical knowledge, I am committed to providing accurate, easy-to-understand content to support pharmacy students and professionals. My aim is to make complex pharmaceutical concepts accessible and useful for real-world application.
Mail- Sachin@pharmacyfreak.com