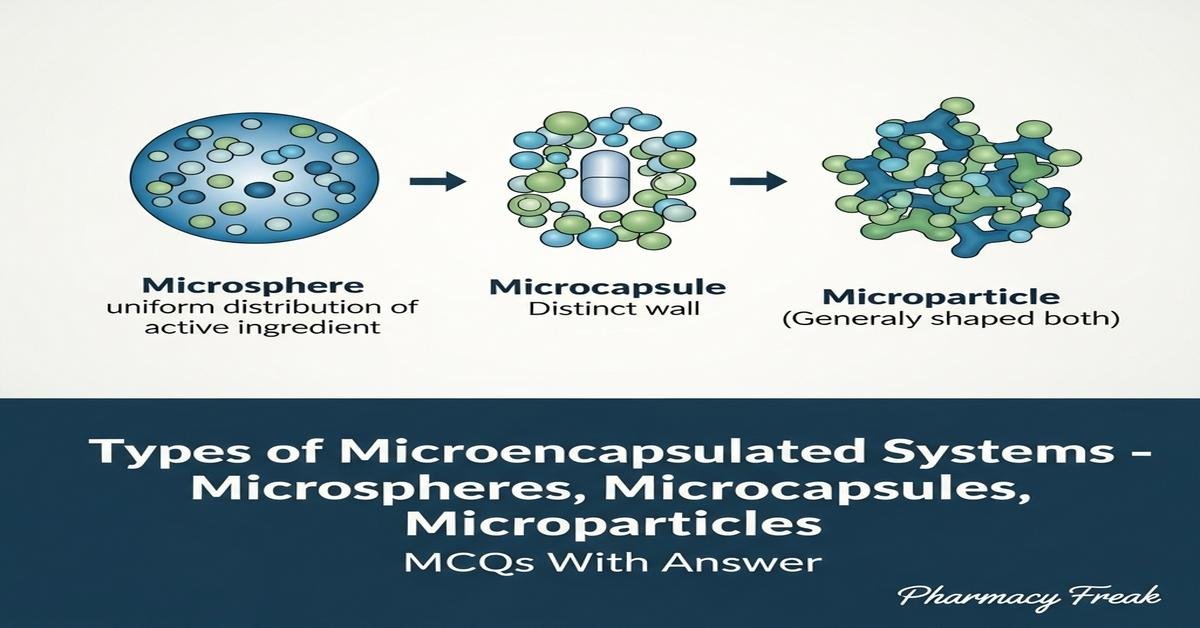
Microencapsulation covers a range of technologies for enclosing drugs within microspheres, microcapsules, and microparticles to control release, improve stability, enable targeted delivery, and modify pharmacokinetics. This overview explains types of microencapsulated systems—matrix and reservoir microspheres, single-core microcapsules, multi-particulate microparticles—plus common polymers (PLGA, ethylcellulose, alginate, chitosan), formulation techniques (spray-drying, solvent evaporation, coacervation, interfacial polymerization, ionic gelation), and key characterization parameters like particle size, encapsulation efficiency, morphology, and release kinetics. Emphasis is on formulation principles, advantages, limitations, and pharmaceutical applications relevant to B. Pharm students preparing for formulation and biopharmaceutics exams. Now let’s test your knowledge with 30 MCQs on this topic.
Q1. Which statement best distinguishes microspheres from microcapsules?
- Microspheres are matrix systems where drug is dispersed throughout a polymer matrix, microcapsules have a core-shell structure.
- Microspheres always have a liquid core and microcapsules always have a solid core.
- Microspheres are larger than microcapsules by definition.
- Microspheres are used only for oral delivery while microcapsules are for parenteral use.
Correct Answer: Microspheres are matrix systems where drug is dispersed throughout a polymer matrix, microcapsules have a core-shell structure.
Q2. The term “microparticles” most accurately refers to:
- A specific reservoir-type particle with a single core and shell.
- A generic term encompassing microspheres and microcapsules in the micrometer range.
- Only particles made from natural polymers like alginate or gelatin.
- Nanoparticles smaller than 100 nm.
Correct Answer: A generic term encompassing microspheres and microcapsules in the micrometer range.
Q3. Which description defines a matrix-type microsphere?
- Drug is confined in a central core surrounded by an impermeable shell.
- Drug molecules are uniformly dispersed throughout a continuous polymer matrix.
- Multiple cores are embedded in a polymeric hydrogel network.
- Particles are hollow and contain gaseous cores for inhalation.
Correct Answer: Drug molecules are uniformly dispersed throughout a continuous polymer matrix.
Q4. PLGA (poly(lactic-co-glycolic acid)) is widely used because it is:
- Non-biodegradable and chemically inert in vivo.
- Biodegradable, biocompatible, and approved for controlled release applications.
- Water-soluble and rapidly cleared from the body.
- Only suitable for oral immediate-release formulations.
Correct Answer: Biodegradable, biocompatible, and approved for controlled release applications.
Q5. Which advantage is typically associated with spray-drying for microencapsulation?
- It always produces particles with perfect core-shell structures.
- It is a single-step, scalable technique suitable for heat-stable drugs and excipients.
- It requires no solvent and is ideal for highly water-soluble drugs.
- It guarantees 100% encapsulation efficiency for all drugs.
Correct Answer: It is a single-step, scalable technique suitable for heat-stable drugs and excipients.
Q6. The solvent evaporation method for preparing microspheres relies primarily on:
- Chemical crosslinking of monomers in a dry state.
- Diffusion of a volatile organic solvent from droplets into an external phase, leaving solid polymer particles.
- Formation of ice crystals that are then lyophilized to produce particles.
- Direct polymerization within an emulsion to form nanogels.
Correct Answer: Diffusion of a volatile organic solvent from droplets into an external phase, leaving solid polymer particles.
Q7. Complex coacervation as a microencapsulation technique is typically based on:
- Phase separation induced by electrostatic interaction between two oppositely charged polymers.
- Spray-freezing of drug solutions to form porous beads.
- Sublimation of volatile components to leave hollow capsules.
- High-pressure homogenization of solid dispersions.
Correct Answer: Phase separation induced by electrostatic interaction between two oppositely charged polymers.
Q8. Interfacial polymerization is most suitable for making which microencapsulated structure?
- Matrix microspheres with uniformly dispersed drug.
- Reservoir-type microcapsules with a polymeric shell formed at an interface.
- Solid lipid nanoparticles via melt emulsification.
- Porous beads produced by gas foaming.
Correct Answer: Reservoir-type microcapsules with a polymeric shell formed at an interface.
Q9. Ionic gelation is commonly used with which natural polymer to form microparticles?
- Polyethylene glycol (PEG)
- Alginate
- Polystyrene
- Polyvinyl chloride (PVC)
Correct Answer: Alginate
Q10. Encapsulation efficiency (EE) refers to:
- The percentage of polymer converted into particles during processing.
- The amount of drug retained within particles relative to the initial drug used in formulation.
- The dissolution rate of the encapsulated drug in simulated gastric fluid.
- The particle yield after drying.
Correct Answer: The amount of drug retained within particles relative to the initial drug used in formulation.
Q11. Drug loading (loading capacity) differs from entrapment efficiency in that loading capacity is:
- The ratio of drug weight to total particle weight, usually expressed as % w/w.
- The percentage of drug that degrades during processing.
- The distribution of particle sizes in a batch.
- The zeta potential measured for charged particles.
Correct Answer: The ratio of drug weight to total particle weight, usually expressed as % w/w.
Q12. Reducing particle size of microspheres generally results in:
- Slower drug release due to decreased surface area.
- Faster drug release due to increased surface area and shorter diffusion paths.
- Complete prevention of burst release phenomena.
- No change in release kinetics for diffusion-controlled drugs.
Correct Answer: Faster drug release due to increased surface area and shorter diffusion paths.
Q13. “Burst release” from microparticles is most commonly caused by:
- Drug chemically bound to polymer backbone.
- Drug adsorbed or loosely associated at or near the particle surface.
- Very low porosity of the particle matrix.
- Complete encapsulation in a thick impermeable shell.
Correct Answer: Drug adsorbed or loosely associated at or near the particle surface.
Q14. Scanning electron microscopy (SEM) is primarily used to evaluate:
- Thermal transitions and crystallinity of the drug-polymer mix.
- Particle surface morphology and shape at high resolution.
- Chemical interactions by identifying functional groups.
- In vitro release kinetics directly.
Correct Answer: Particle surface morphology and shape at high resolution.
Q15. Why is zeta potential important for microparticles?
- It quantifies thermal stability of the polymer matrix.
- It predicts colloidal stability and tendency for particle aggregation.
- It measures the drug loading directly.
- It indicates the exact porosity of each particle.
Correct Answer: It predicts colloidal stability and tendency for particle aggregation.
Q16. Differential scanning calorimetry (DSC) helps in microencapsulation studies by:
- Determining particle size distribution in suspensions.
- Identifying thermal transitions that indicate drug crystallinity or polymer-drug interactions.
- Measuring surface charge and ionic strength.
- Visualizing internal core-shell structures directly.
Correct Answer: Identifying thermal transitions that indicate drug crystallinity or polymer-drug interactions.
Q17. Fourier-transform infrared spectroscopy (FTIR) is useful to detect:
- Changes in chemical functional groups suggesting drug–polymer interactions.
- Particle mechanical strength under compression.
- Exact percentage of encapsulation efficiency without extraction.
- In vivo biodegradation rates.
Correct Answer: Changes in chemical functional groups suggesting drug–polymer interactions.
Q18. Which sterilization method is often preferred for polymeric microparticles when heat sterilization would degrade the polymer?
- Autoclaving at 121°C for 15 minutes.
- Gamma irradiation at appropriate dose.
- Dry heat sterilization at 200°C.
- Sterile filtration through 0.22 µm filters of the final dry powder.
Correct Answer: Gamma irradiation at appropriate dose.
Q19. For pulmonary delivery of microspheres, the aerodynamic diameter generally desired for deep lung deposition is:
- Greater than 10 µm.
- 1–5 µm.
- 100–200 µm.
- Less than 0.1 µm.
Correct Answer: 1–5 µm.
Q20. Which polymer is commonly used to impart mucoadhesive properties to microencapsulated systems?
- Polystyrene
- Chitosan
- Polyethylene
- Polymethyl methacrylate (PMMA)
Correct Answer: Chitosan
Q21. Which of the following is a biodegradable synthetic polymer frequently used for sustained-release microspheres?
- Poly(lactic-co-glycolic acid) (PLGA)
- Polystyrene
- Polyvinyl acetate
- Polyethylene
Correct Answer: Poly(lactic-co-glycolic acid) (PLGA)
Q22. Increasing polymer hydrophobicity in a microsphere formulation typically results in:
- Faster drug release due to improved water penetration.
- Slower drug release due to reduced water uptake and slower polymer degradation.
- No change to release profile for hydrophobic drugs.
- Complete prevention of enzymatic degradation in vivo.
Correct Answer: Slower drug release due to reduced water uptake and slower polymer degradation.
Q23. Which mathematical model is commonly applied to describe diffusion-controlled drug release from planar systems and often used for microparticles in early approximation?
- Michaelis-Menten kinetics
- Higuchi model
- Arrhenius equation
- Langmuir isotherm
Correct Answer: Higuchi model
Q24. High porosity in microparticles is most likely to cause which effect on drug release?
- Reduced initial burst and much slower overall release.
- Increased initial burst release and faster overall release.
- No effect on release if the drug is hydrophobic.
- Complete drug retention with no release.
Correct Answer: Increased initial burst release and faster overall release.
Q25. Which method typically forms a polymer shell at the oil–water interface to encapsulate an oily core?
- Solvent evaporation in a single aqueous phase without interface control.
- Interfacial polymerization.
- Spray drying from a pure aqueous solution.
- Freeze–thaw cycling of a bulk dispersion.
Correct Answer: Interfacial polymerization.
Q26. A major advantage of microencapsulation for labile drugs is:
- Guaranteed elimination of all impurities.
- Improved chemical and physical stability by protecting the drug from environmental factors.
- Immediate and complete absorption in the GI tract.
- Transformation of hydrophilic drugs into highly lipophilic forms.
Correct Answer: Improved chemical and physical stability by protecting the drug from environmental factors.
Q27. For encapsulating a hydrophilic protein drug, which emulsion technique is most appropriate?
- Oil-in-water (o/w) single emulsion with hydrophobic drug in oil phase.
- Water-in-oil-in-water (w/o/w) double emulsion to retain hydrophilic drug in internal aqueous phase.
- Direct melt dispersion in molten polymer without aqueous phases.
- Gas-assisted foaming to entrap protein in gas pockets.
Correct Answer: Water-in-oil-in-water (w/o/w) double emulsion to retain hydrophilic drug in internal aqueous phase.
Q28. Which statement correctly differentiates nanospheres from microspheres?
- Nanospheres are typically smaller (nano-scale) and may have different biodistribution and cellular uptake compared to microspheres.
- Nanospheres always contain a gaseous core while microspheres do not.
- Microspheres are always biodegradable while nanospheres are not.
- Nanospheres cannot be characterized by SEM due to size limitations.
Correct Answer: Nanospheres are typically smaller (nano-scale) and may have different biodistribution and cellular uptake compared to microspheres.
Q29. Which fabrication approach is known for producing microparticles with very narrow and controllable size distributions?
- Conventional batch solvent evaporation with simple stirring.
- Microfluidic droplet generation techniques.
- Bulk freeze-drying without droplet control.
- Random spray-drying without nozzle control.
Correct Answer: Microfluidic droplet generation techniques.
Q30. In regulatory terms, what is a critical quality concern for solvent-based microencapsulation processes?
- Presence of residual organic solvents beyond ICH Q3C limits.
- Exclusively particle color uniformity.
- Guarantee of zero degradation of all excipients after 50 years.
- Mandatory use of natural polymers only.
Correct Answer: Presence of residual organic solvents beyond ICH Q3C limits.

I am a Registered Pharmacist under the Pharmacy Act, 1948, and the founder of PharmacyFreak.com. I hold a Bachelor of Pharmacy degree from Rungta College of Pharmaceutical Science and Research. With a strong academic foundation and practical knowledge, I am committed to providing accurate, easy-to-understand content to support pharmacy students and professionals. My aim is to make complex pharmaceutical concepts accessible and useful for real-world application.
Mail- Sachin@pharmacyfreak.com