Monobactams – chemistry and activity MCQs With Answer
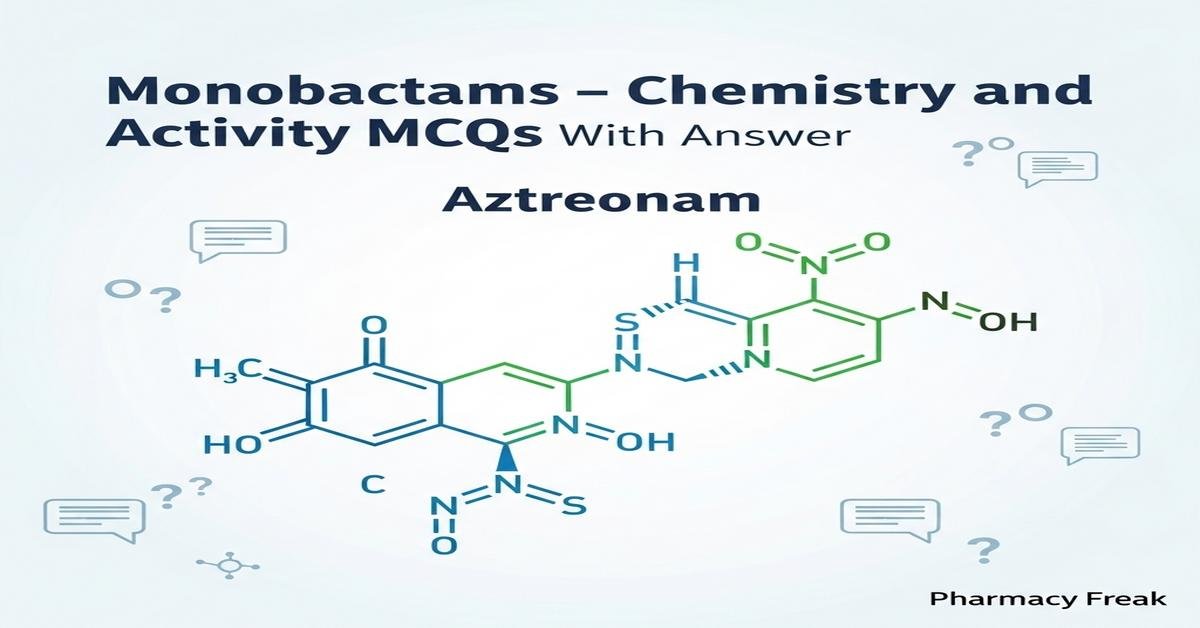
Monobactams are a distinct class of monocyclic β-lactam antibiotics characterized by a single β-lactam ring and unique side chains that determine their spectrum, stability, and pharmacokinetics. Aztreonam is the prototype, notable for potent activity against aerobic Gram-negative bacilli including Pseudomonas and relative stability to many β-lactamases. Understanding monobactam chemistry—ring structure, side-chain modifications, and mechanisms of β-lactamase resistance—helps predict clinical activity, toxicities, and drug interactions. This concise, keyword-rich introduction prepares B.Pharm students for focused practice on synthesis, mechanism of action, spectrum, resistance, pharmacokinetics, and adverse effects. Now let’s test your knowledge with 30 MCQs on this topic.
Q1. Which structural feature defines monobactams?
- A fused bicyclic β-lactam core
- A single (monocyclic) β-lactam ring without a fused thiazolidine or dihydrothiazine
- A macrocyclic lactone ring
- A cephalosporin-like dihydrothiazine ring
Correct Answer: A single (monocyclic) β-lactam ring without a fused thiazolidine or dihydrothiazine
Q2. The clinically important monobactam available commercially is:
- Imipenem
- Aztreonam
- Meropenem
- Clavulanic acid
Correct Answer: Aztreonam
Q3. Monobactams primarily act by inhibiting which bacterial target?
- DNA gyrase
- Protein synthesis at 30S ribosome
- Peptidoglycan cross-linking by binding penicillin-binding proteins (PBPs)
- Folic acid synthesis
Correct Answer: Peptidoglycan cross-linking by binding penicillin-binding proteins (PBPs)
Q4. Aztreonam shows best antibacterial activity against:
- Gram-positive cocci
- Anaerobes including Bacteroides
- Aerobic Gram-negative rods such as Pseudomonas and Enterobacteriaceae
- Mycobacteria
Correct Answer: Aerobic Gram-negative rods such as Pseudomonas and Enterobacteriaceae
Q5. Compared to penicillins and cephalosporins, monobactams generally have:
- Broad Gram-positive coverage
- Significant anaerobic activity
- Minimal cross-allergenicity with penicillins in most patients
- Oral bioavailability similar to amoxicillin
Correct Answer: Minimal cross-allergenicity with penicillins in most patients
Q6. The resistance of some β-lactamases to hydrolyze monobactams is primarily due to:
- Complete inability of monobactams to access PBPs
- Steric hindrance from side chains that decrease β-lactamase binding
- Inhibition of β-lactamases by monobactams
- Monobactam activation of efflux pumps
Correct Answer: Steric hindrance from side chains that decrease β-lactamase binding
Q7. Aztreonam is often chosen for patients with penicillin allergy because:
- It is an aminoglycoside
- It has a monocyclic structure with low IgE cross-reactivity to penicillin
- It is inactivated by the same antibodies as penicillin
- It blocks histamine release caused by penicillin
Correct Answer: It has a monocyclic structure with low IgE cross-reactivity to penicillin
Q8. The primary route of administration for aztreonam in systemic infections is:
- Oral tablets
- Topical cream
- Intravenous or intramuscular injection
- Inhaled aerosol only
Correct Answer: Intravenous or intramuscular injection
Q9. Aztreonam’s stability to many β-lactamases means it is:
- Completely immune to all β-lactamases including ESBLs and carbapenemases
- Resistant to some penicillinases but may be hydrolyzed by certain extended-spectrum β-lactamases (ESBLs)
- Only active when combined with clavulanic acid
- Inactive against Gram-negative bacteria
Correct Answer: Resistant to some penicillinases but may be hydrolyzed by certain extended-spectrum β-lactamases (ESBLs)
Q10. The mechanism by which monobactams produce bacterial cell death is best described as:
- Induction of lethal DNA breaks
- Inhibition of transpeptidase activity leading to weakened cell wall and lysis
- Disruption of cytoplasmic membrane potential directly
- Competitive inhibition of folate synthesis enzymes
Correct Answer: Inhibition of transpeptidase activity leading to weakened cell wall and lysis
Q11. Which penicillin-binding protein is a primary target for aztreonam in many Gram-negative bacteria?
- PBP-1 only
- PBP-2 only
- PBP-3 (associated with septation)
- PBP-4 exclusively
Correct Answer: PBP-3 (associated with septation)
Q12. A clinically relevant inhaled formulation of aztreonam is used for:
- Tuberculosis treatment
- Cystic fibrosis patients with Pseudomonas aeruginosa lung infection
- Systemic sepsis
- Topical skin infections
Correct Answer: Cystic fibrosis patients with Pseudomonas aeruginosa lung infection
Q13. The chemical modification most responsible for altering spectrum and β-lactamase stability in monobactams is:
- Changes in the macrocyclic ring size
- Substitutions on the β-lactam nitrogen and C3/C4 side chains
- Glycosylation of the β-lactam core
- Conjugation to aminoglycosides
Correct Answer: Substitutions on the β-lactam nitrogen and C3/C4 side chains
Q14. Which adverse effect is commonly associated with parenteral aztreonam therapy?
- Ototoxicity like aminoglycosides
- Severe nephrotoxicity in all patients
- Gastrointestinal upset and injection site reactions
- Irreversible bone marrow aplasia
Correct Answer: Gastrointestinal upset and injection site reactions
Q15. Monobactams are classified pharmacodynamically as:
- Concentration-dependent killers
- Time-dependent (time above MIC) killers
- AUC/MIC dependent like fluoroquinolones
- Non-antibacterial immunomodulators
Correct Answer: Time-dependent (time above MIC) killers
Q16. Cross-reactivity between aztreonam and ceftazidime can occur due to:
- Shared identical β-lactam cores
- Similar side-chain structures leading to antibody recognition
- Both being aminoglycosides
- Both being inactivated by clavulanic acid
Correct Answer: Similar side-chain structures leading to antibody recognition
Q17. In medicinal chemistry, replacing a side chain on a monobactam primarily aims to:
- Convert it into a macrolide
- Alter spectrum, β-lactamase stability, and pharmacokinetics
- Increase protein synthesis inhibition
- Remove the β-lactam ring entirely
Correct Answer: Alter spectrum, β-lactamase stability, and pharmacokinetics
Q18. Which laboratory test helps determine susceptibility of Gram-negative isolates to aztreonam?
- Kirby-Bauer disk diffusion or MIC determination using aztreonam disks or strips
- Rapid antigen detection for Streptococcus
- Acid-fast staining
- Serology for β-lactam antibodies
Correct Answer: Kirby-Bauer disk diffusion or MIC determination using aztreonam disks or strips
Q19. The primary elimination route for aztreonam is:
- Hepatic metabolism with extensive CYP450 involvement
- Renal excretion as unchanged drug
- Excretion in bile only
- Metabolism to active metabolites eliminated in feces
Correct Answer: Renal excretion as unchanged drug
Q20. Which beta-lactamase type is most likely to hydrolyze monobactams and render them ineffective?
- Class A narrow-spectrum penicillinases only
- Extended-spectrum β-lactamases (ESBLs) and some carbapenemases
- Only metalloenzymes with zinc
- Only human proteases
Correct Answer: Extended-spectrum β-lactamases (ESBLs) and some carbapenemases
Q21. The term “monobactam” refers to:
- An antibiotic with two fused rings like penicillins
- A single-ring β-lactam antibiotic
- A macrolide with a mono-glycoside
- A sulfonamide derivative
Correct Answer: A single-ring β-lactam antibiotic
Q22. Which clinical use is most appropriate for aztreonam?
- Empiric therapy for community-acquired MRSA skin infection
- Targeted therapy for severe infections due to Gram-negative rods in penicillin-allergic patients
- Treatment of Clostridioides difficile colitis
- Primary therapy for atypical pneumonia
Correct Answer: Targeted therapy for severe infections due to Gram-negative rods in penicillin-allergic patients
Q23. In drug design, the oxime and sulfamoyl groups on aztreonam influence:
- Only taste and color
- Spectrum, β-lactamase resistance, and PBP binding
- Conversion to penicillin inside bacteria
- Ability to phosphorylate bacterial proteins
Correct Answer: Spectrum, β-lactamase resistance, and PBP binding
Q24. Which statement about monobactam oral availability is correct?
- Aztreonam is well absorbed orally and commonly given as tablets
- Most monobactams are poorly absorbed and are administered parenterally
- Monobactams are only administered topically
- All monobactams are prodrugs activated in the stomach
Correct Answer: Most monobactams are poorly absorbed and are administered parenterally
Q25. A pharmacokinetic advantage of monobactams in renal impairment requires:
- No dose adjustment ever
- Careful dose adjustment due to primary renal excretion
- Addition of CYP450 inhibitors
- Switch to oral therapy only
Correct Answer: Careful dose adjustment due to primary renal excretion
Q26. Which of the following best explains why monobactams lack activity against Gram-positive cocci?
- Inability to penetrate the outer membrane characteristic of Gram-negative bacteria
- Poor affinity of monobactams for PBPs of many Gram-positive organisms and inability to reach target effectively
- Inhibition of protein synthesis rather than cell wall synthesis
- Exclusive binding to ribosomal RNA
Correct Answer: Poor affinity of monobactams for PBPs of many Gram-positive organisms and inability to reach target effectively
Q27. In structural comparison, monobactams differ from carbapenems by:
- Monobactams have a monocyclic β-lactam; carbapenems have a bicyclic structure and a different ring heteroatom
- Monobactams are macrolides
- Carbapenems lack a β-lactam ring entirely
- Monobactams contain a glycopeptide moiety
Correct Answer: Monobactams have a monocyclic β-lactam; carbapenems have a bicyclic structure and a different ring heteroatom
Q28. Which laboratory finding would suggest aztreonam therapy is appropriate?
- Isolate identified as methicillin-resistant Staphylococcus aureus
- Isolate identified as Pseudomonas aeruginosa susceptible to aztreonam
- Blood culture positive for anaerobic cocci only
- Culture shows typical oral streptococci sensitive to penicillin
Correct Answer: Isolate identified as Pseudomonas aeruginosa susceptible to aztreonam
Q29. Which drug interaction is most relevant for aztreonam?
- Potentiation of nephrotoxicity with aminoglycosides when used concurrently
- Reduced effect when given with vitamin K
- Inactivation by proton pump inhibitors
- Enhanced CYP3A4 metabolism
Correct Answer: Potentiation of nephrotoxicity with aminoglycosides when used concurrently
Q30. From a pharmaceutical chemistry perspective, improving monobactam oral bioavailability would likely require:
- Increasing hydrophilicity to prevent absorption
- Structural modifications to enhance membrane permeability and stability to gastric acid
- Removing the β-lactam ring to avoid degradation
- Converting it into a peptide antibiotic
Correct Answer: Structural modifications to enhance membrane permeability and stability to gastric acid

I am a Registered Pharmacist under the Pharmacy Act, 1948, and the founder of PharmacyFreak.com. I hold a Bachelor of Pharmacy degree from Rungta College of Pharmaceutical Science and Research. With a strong academic foundation and practical knowledge, I am committed to providing accurate, easy-to-understand content to support pharmacy students and professionals. My aim is to make complex pharmaceutical concepts accessible and useful for real-world application.
Mail- Sachin@pharmacyfreak.com