Fats and oils.
Fats and oils are present in the majority of the liquid present in the adipose tissue of mammals. Fats and oils are mainly the glyceryl esters of various fatty acids like palmitic, stearic, linoleic, and linolenic.
They are also called triglycerides as three molecules of fatty condense with one mole of glycerol to form fat.
Define fats?
The compounds that can dissolve in organic solvents and could not dissolve in water are called fats. Fats are generally solid at room temperature
Solid fats can be classified into two groups
- 1) Saturated fat
- 2) Trans fat
1) Saturated fat – it is also known as solid fat. Saturated fat is found more in animal fat or red meat than compared to fish and poultry. Some tropical oil also has saturated fats for example cocoa butter, coconut oil, palm oil, etc.
Saturated fats are found mainly in non-dairy products and snacks such as butter, cakes, and cookies containing food that contains large amounts of saturated fats.
2) Trans-fat- Trans-fat is fat, which is changed at the molecular level to increase its shelf life. The process that is used to make trans-fat is known as hydrogenation. This fat is harder at room temperature.
- Trans-fat is generally used in making crispier food items.
- It is found in large amounts in processed food such as cookies, chips, etc.
- We should avoid eating food containing trans-fat because cholesterol level is increased by it.
Define oils?
Fats, which exist in liquid form at room temperature, are known as oils. Oils are fats that are unsaturated in other words saturated fats are called oil.
Unsaturated fats can be classified into two categories.
- 1) Monounsaturated fats
- 2) Polyunsaturated fats
1) Monounsaturated fats– avocado, nuts, and vegetable oils are the source of Monounsaturated fats. Monounsaturated fats help in lowering cholesterol levels which is why it is considered good for health.
2) Polyunsaturated fats- it is found in oils such as corn, soybean, and sunflower. It is also obtained from seafood. It is also helpful in lowering cholesterol levels.
Fats and oils are of two types-
a) Simple
b) Mixed
a) Simple– In simple fats and oils, all the 3 fatty acids of triglycerides are identical.
b) Mixed– In mixed fats and oils, all the three fatty acids of triglyceride are not identical.
Natural fats are mainly mixed glycerides and they do not have free acid or base groups so they are also known as neutral fats. Fats and oils are obtained from plants as well as from animals. For example lord (pig fat), tallow (beef), coconut oil, castor oil, olive oil, soybean oil, etc. Fats exist in animals under the skin and between the muscular tissue. In plants, fats occur in seeds, in fruits.
A crude fat along with glyceryl ester contains some amount of free fatty acids and 1-2% of unsaponifiable matter like Sterols.
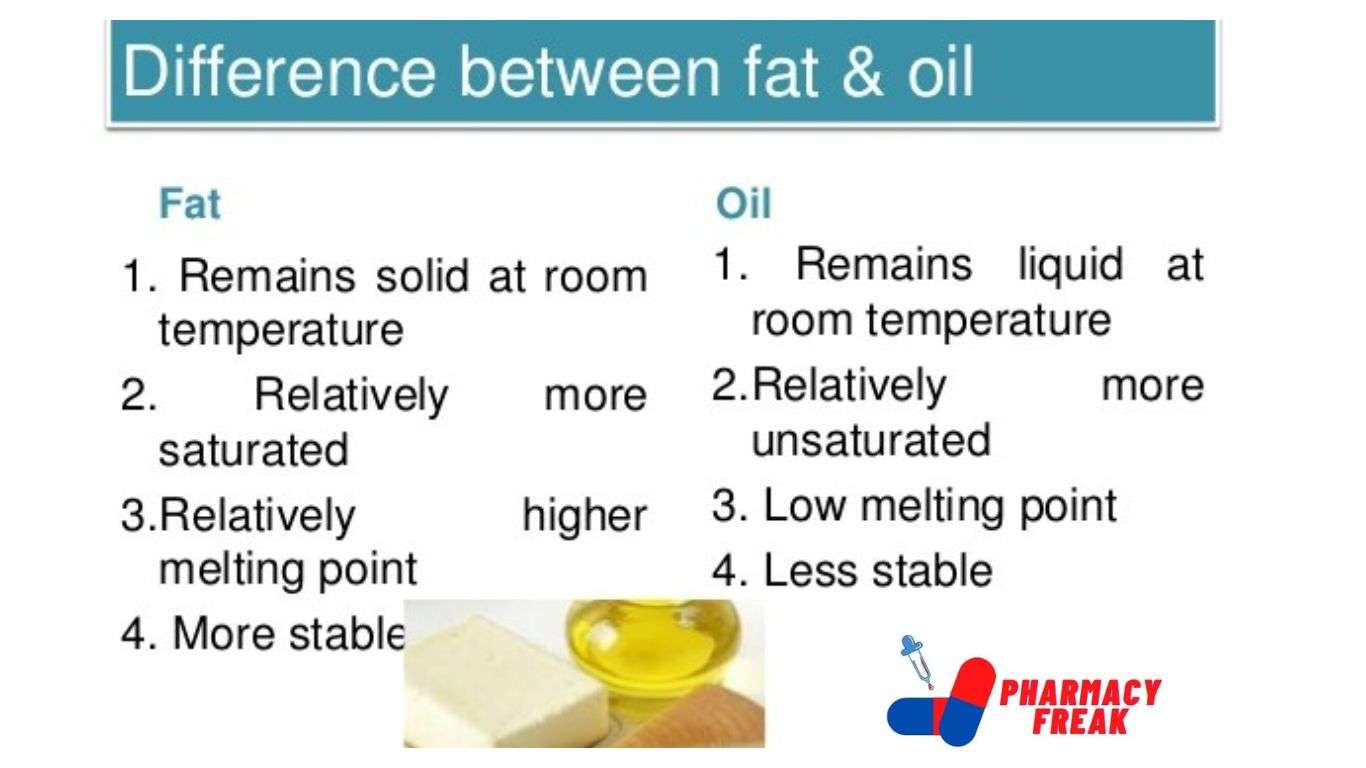
Difference between fats and oils
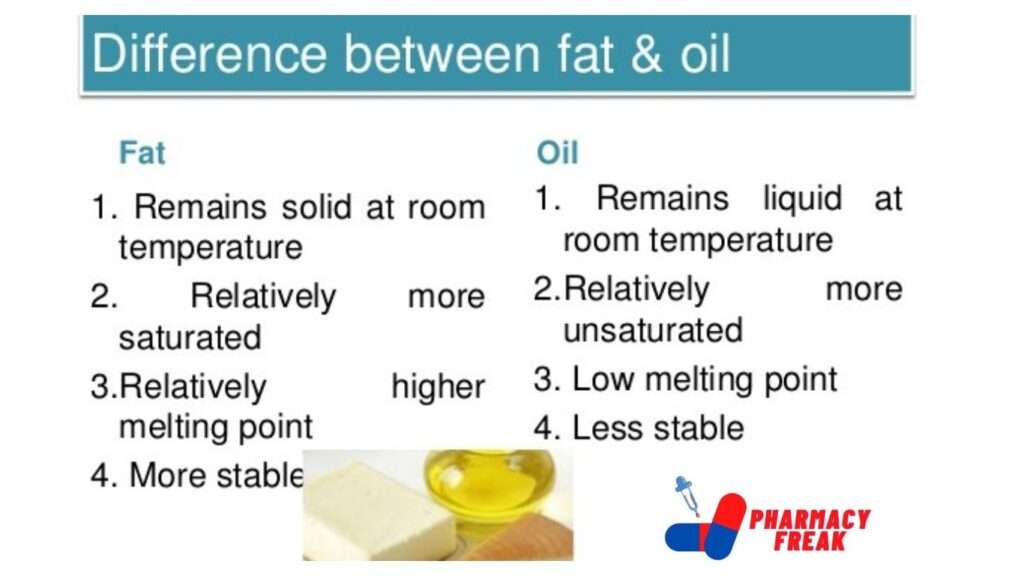
| Fats | Oils |
| Fats are solids or semi-solids at room temperature | Oils are liquids at room temperature |
| Fats contain large amounts of saturated fatty acids example stearic and palmitic acid | oils contain a large amount of unsaturated acids example oleic acid |
| Fats melt at a higher temperature | Oil Melts at a lower temperature |
| Fats are generally obtained from animals | Oils are vegetable fats |
| Fats do not contain double bond | Oils have double bond |
| Fats are more stable | Oils are less stable |
Nomenclature of fats
The main part of fat is glycerol, and glycerol is trihydric alcohol and its triester has three acid residues. If all three residues are identical, it is known as simple fat and if they are not identical then the fat is called mixed glyceride. For simple fat, the name is given by naming the alcohol (glycerol) or its radical (glyceryl) and naming the acid.
Physical and chemical properties of fat
Fats and oils are colorless or pale yellow.
These are insoluble in water and polar solvents but soluble in nonpolar solvents such as ether, carbon tetrachloride, and carbon disulfide.
Various chemical reactions of fats and oils
1. Hydrolysis
Sodium hydroxide causes hydrolysis of fats and it results in cleavage of ester linkage of fats to give glycerol and sodium salts of long-chain fatty acids known as soaps.
Releases can also be done by heating fat with water under pressure. Alkaline hydrolysis of fats produces salts of fatty acids called soaps and hence this reaction is also known as saponification. Common soaps are the mixture of sodium salts of C-atoms (12 atoms) and higher fatty acids.
Soap molecules have both lipophilic (lipid living) and hydrophilic (water-loving) groups. The lipophilic group dissolves oil while the hydrophilic portion dissolves water. Soap molecules on dissolution in water form micelle.
Hydrolysis reaction can be done by 3 ways-
a) By water–
Fat undergoes hydrolysis in presence of water at 443 k and 6 – 8 atmospheric pressure. Zinc oxide is used as a catalyst.
b) By enzymes–
hydrolysis of fats and oils can be done by adding enzyme lipase to an emulsion of fat in water.
c) By acids–
Mineral acids cause the hydrolysis of fats. For this mixture of sulphonic acids which are obtained by sulphonation of mixture of oleic acid and benzene. The cleansing property of soap depends upon the ability to form an emulsion with fat-soluble materials.
2. Hydrogenation
Oils have a large amount of unsaturated portion in the form of glycerides. When hydrogen is passed through oils under pressure and by using catalyst at high-temperature oils get converted into solid fats. This process is also known as the hardening of oil. By hydrogenation, the unsaturated acid part of oil gets reduced into the saturated part and hence liquid oil gets converted into semi-solid fat. Hydrogenation is done effectively by adding a small amount of finely divided Nickle or Raney Nickle as a catalyst.
In actuality, hydrogenation is not carried out completely but the process is stopped as the fat of desired consistency and viscosity is obtained. Fully hydrogenated fats are very hard and cannot be digested easily in the intestine. Hydrogenation Converse only a part of the glycerides of unsaturated acids into situated acids. This property is used in the production of Margarine and vegetable ghee.
3. Hydrogenolysis
This is a cleavage reaction in which a fat or oil molecule is treated with excess hydrogen under pressure in presence of a copper chromium catalyst. In this reaction fat or oil gets splits up into glycerol and higher aliphatic alcohols.
Hydrogen releases mean splitting of a given compound using hydrogen sometimes, active hydrogen can be obtained by reaction of metallic sodium with water-insoluble alcohol like 2-hexanol.
4. Saponification
When hydrolysis of fats and oils is carried out by using alkalies like causative soda or causative potash alkaline salts of fatty acids known as soaps and glycerol are obtained. This process is known as saponification.
5. Rancidification
On long storage and in contact with air, moisture, and sunlight, oils and fats undergo decomposition and start smelling unpleasant. This process is known as rancidification. Oil is said to be rancid oil.
Rancidity occurs by the following causes
- a) oxidation of unsaturated acids:
- b) enzymatic hydrolysis
- C) beta-oxidation of situated fatty acid
- 6. Drawing oils
Some glycerides of unsaturated acids having two or more double bonds absorb oxygen from the air and get polymerized to form a hard transparent coating which is used in making paints and oilcloth. This phenomenon is called drying and oils as drying oils.
For example, linseed oil, Tung oil, and perilla oil are drying oil.
MCQ/ Fats and Oils Pharmaceutical Organic Chemistry 3rd semester B.Pharmacy

I am a Registered Pharmacist under the Pharmacy Act, 1948, and the founder of PharmacyFreak.com. I hold a Bachelor of Pharmacy degree from Rungta College of Pharmaceutical Science and Research. With a strong academic foundation and practical knowledge, I am committed to providing accurate, easy-to-understand content to support pharmacy students and professionals. My aim is to make complex pharmaceutical concepts accessible and useful for real-world application.
Mail- Sachin@pharmacyfreak.com