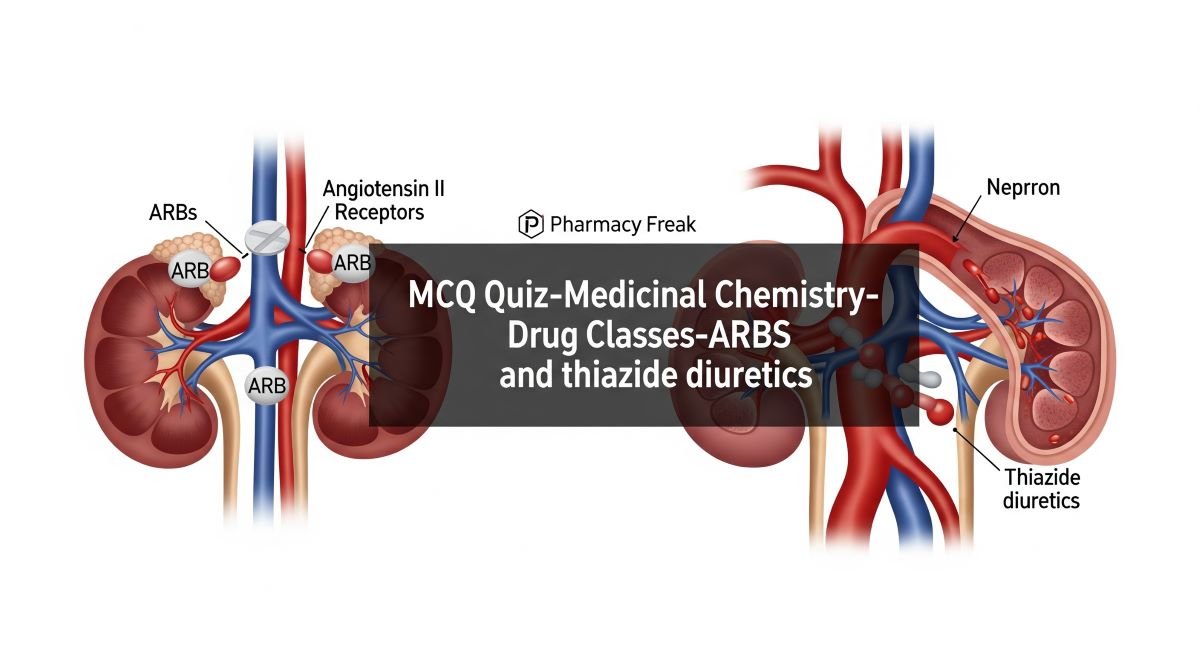
Angiotensin II Receptor Blockers (ARBs) and Thiazide Diuretics are fundamental drug classes in the management of hypertension and other cardiovascular conditions. ARBs offer a targeted approach to inhibiting the renin-angiotensin-aldosterone system, while thiazide diuretics effectively reduce blood volume and vascular resistance. For PharmD students, a comprehensive understanding of the medicinal chemistry of these agents—including their structure-activity relationships (SAR), mechanisms of action, pharmacokinetic properties, and metabolic pathways—is essential for optimizing patient care and ensuring safe and effective pharmacotherapy. This MCQ quiz will explore the key medicinal chemistry aspects of both ARBs and Thiazide Diuretics.
1. Angiotensin II Receptor Blockers (ARBs) primarily exert their antihypertensive effect by selectively blocking which receptor subtype?
- A. AT2 receptor
- B. AT1 receptor
- C. Bradykinin B2 receptor
- D. Aldosterone receptor
Answer: B. AT1 receptor
2. Which structural moiety is a common pharmacophoric feature in many ARBs, responsible for mimicking the C-terminal carboxylate or a tyrosine-like phenol of Angiotensin II?
- A. A sulfonylurea group
- B. A biphenyltetrazole system
- C. A dihydropyridine ring
- D. A catecholamine structure
Answer: B. A biphenyltetrazole system
3. Losartan, the first orally active non-peptide ARB, contains which acidic group that is crucial for its activity?
- A. A sulfhydryl group
- B. A carboxylic acid group on the imidazole ring (after metabolism)
- C. A tetrazole ring (acidic isostere)
- D. A phosphonate group
Answer: C. A tetrazole ring (acidic isostere)
4. The active metabolite of Losartan, responsible for a significant portion of its antihypertensive effect, is formed by:
- A. Hydrolysis of an ester group
- B. Oxidation of a hydroxymethyl group to a carboxylic acid (EXP3174)
- C. N-dealkylation
- D. Glucuronidation
Answer: B. Oxidation of a hydroxymethyl group to a carboxylic acid (EXP3174)
5. Valsartan is an ARB that differs from Losartan structurally. Valsartan achieves its acidic character primarily through:
- A. A tetrazole ring
- B. A carboxylic acid group
- C. A sulfonate group
- D. A phenolic hydroxyl group
Answer: B. A carboxylic acid group
6. Candesartan cilexetil is a prodrug form of which active ARB?
- A. Valsartan
- B. Candesartan
- C. Irbesartan
- D. Olmesartan
Answer: B. Candesartan
7. The conversion of Candesartan cilexetil to Candesartan in the body involves primarily which type of metabolic reaction?
- A. Oxidation
- B. Reduction
- C. Hydrolysis of an ester group
- D. Methylation
Answer: C. Hydrolysis of an ester group
8. Irbesartan is characterized by which heterocyclic ring system fused to the imidazole ring?
- A. A triazole ring
- B. A cyclopentane ring forming a spiro compound
- C. A benzimidazole structure
- D. A pyrimidine ring
Answer: B. A cyclopentane ring forming a spiro compound
9. Telmisartan is known for its very long half-life among ARBs. This is partly attributed to its:
- A. High water solubility
- B. Rapid metabolism to multiple active metabolites
- C. High lipophilicity and extensive plasma protein binding
- D. Exclusive renal excretion
Answer: C. High lipophilicity and extensive plasma protein binding
10. Olmesartan medoxomil is a prodrug. The “medoxomil” portion is designed to:
- A. Increase potency at the AT1 receptor
- B. Enhance oral bioavailability through ester hydrolysis
- C. Reduce the incidence of cough
- D. Provide a secondary diuretic effect
Answer: B. Enhance oral bioavailability through ester hydrolysis
11. Compared to ACE inhibitors, a key advantage of ARBs regarding side effect profile is a lower incidence of:
- A. Hyperkalemia
- B. First-dose hypotension
- C. Dry cough and angioedema
- D. Renal impairment
Answer: C. Dry cough and angioedema
12. The biphenyl part of the biphenyltetrazole moiety in many ARBs is crucial for:
- A. Binding directly to the zinc ion in ACE (which ARBs do not inhibit)
- B. Providing the correct spatial orientation for the tetrazole and other groups to interact with the AT1 receptor
- C. Increasing water solubility
- D. Facilitating renal excretion
Answer: B. Providing the correct spatial orientation for the tetrazole and other groups to interact with the AT1 receptor
13. The tetrazole ring (or a carboxylic acid) in ARBs is designed to mimic which amino acid residue of Angiotensin II?
- A. Proline
- B. Histidine
- C. Phenylalanine (its C-terminal carboxylate) or Tyrosine (its phenolic OH)
- D. Valine
Answer: C. Phenylalanine (its C-terminal carboxylate) or Tyrosine (its phenolic OH)
14. Eprosartan is structurally different from many other ARBs as it was designed as a mimic of:
- A. Losartan
- B. Angiotensin II’s C-terminal region directly
- C. Bradykinin
- D. Teprotide
Answer: B. Angiotensin II’s C-terminal region directly
15. Which ARB has a dual mode of action, including PPAR-gamma agonism, although this is not its primary approved indication?
- A. Losartan
- B. Valsartan
- C. Telmisartan
- D. Candesartan
Answer: C. Telmisartan
16. The imidazole or imidazoline-like ring found in ARBs like Losartan and Irbesartan is important for:
- A. Binding to the zinc ion
- B. Mimicking the imidazole ring of Histidine in Angiotensin II
- C. Undergoing metabolic oxidation
- D. Prodrug hydrolysis
Answer: B. Mimicking the imidazole ring of Histidine in Angiotensin II
17. Azilsartan medoxomil is a prodrug of Azilsartan. Azilsartan is characterized by which unique heterocyclic ring in its structure?
- A. A benzimidazole-7-carboxylic acid
- B. A simple imidazole ring
- C. A tetrazole ring directly attached to the biphenyl
- D. An oxadiazole ring
Answer: D. An oxadiazole ring
18. The general SAR for ARBs suggests that the acidic group (tetrazole or COOH) is essential for:
- A. Oral absorption
- B. Strong ionic interaction with the AT1 receptor
- C. Preventing metabolism
- D. Reducing protein binding
Answer: B. Strong ionic interaction with the AT1 receptor
19. Which of these ARBs is NOT a prodrug and is administered in its active form?
- A. Candesartan cilexetil
- B. Olmesartan medoxomil
- C. Valsartan
- D. Azilsartan medoxomil
Answer: C. Valsartan
20. The lipophilic side chains in ARBs, such as the n-butyl group in Losartan or the n-propyl group in Irbesartan, are designed to interact with which region of the AT1 receptor?
- A. A polar, hydrophilic pocket
- B. A specific metal ion binding site
- C. A hydrophobic pocket
- D. The extracellular N-terminal domain
Answer: C. A hydrophobic pocket
21. The advantage of ARBs blocking the AT1 receptor directly, rather than inhibiting ACE, is that:
- A. It allows for the formation of Angiotensin II via non-ACE pathways to still be blocked at the receptor level.
- B. It completely prevents bradykinin formation.
- C. It has a much faster onset of action.
- D. It leads to a greater reduction in aldosterone.
Answer: A. It allows for the formation of Angiotensin II via non-ACE pathways to still be blocked at the receptor level.
22. The stereochemistry of the chiral centers in ARBs like Valsartan is important for:
- A. Water solubility only
- B. Proper fit and optimal binding to the AT1 receptor
- C. Prodrug activation
- D. Preventing metabolism
Answer: B. Proper fit and optimal binding to the AT1 receptor
23. Many ARBs are marketed as single enantiomers or are achiral. Losartan, for example, has a chiral center formed after metabolism to EXP3174. The active EXP3174 is:
- A. Racemic
- B. Primarily the (S)-enantiomer
- C. Primarily the (R)-enantiomer
- D. A mixture of diastereomers
Answer: C. Primarily the (R)-enantiomer (Note: EXP3174 itself is achiral if referring to the main active metabolite, but Losartan’s activity involves this specific transformation. The question phrasing could be tricky; however, many ARBs themselves are chiral, like Valsartan, and specific stereochemistry is crucial.) Correction: Losartan itself is achiral. The metabolite EXP3174 is the carboxylic acid. The key chiral center in many related ARBs if present dictates activity. For ARBs with inherent chirality, specific enantiomers are active. For Losartan, it is achiral, its metabolite EXP3174 has the activity. Let’s rephrase or replace this question to be clearer about ARB stereochemistry in general or a specific chiral ARB. Revisiting question for accuracy with common ARBs like Valsartan: 23. Valsartan possesses a chiral center. The therapeutically active form is:
- A. The (R)-enantiomer
- B. The (S)-enantiomer
- C. A racemic mixture
- D. A meso compound
Answer: B. The (S)-enantiomer
24. The development of ARBs was a progression from peptide antagonists of Angiotensin II. A major challenge with early peptide antagonists was:
- A. Lack of specificity for AT1 receptors
- B. Poor oral bioavailability and short duration of action
- C. Excessive potency leading to hypotension
- D. Inability to cross cell membranes
Answer: B. Poor oral bioavailability and short duration of action
25. The design strategy for non-peptide ARBs often involved finding small molecules that could mimic the key interactions of:
- A. Renin with angiotensinogen
- B. Angiotensin I with ACE
- C. Angiotensin II with the AT1 receptor
- D. Aldosterone with its mineralocorticoid receptor
Answer: C. Angiotensin II with the AT1 receptor
26. Thiazide diuretics primarily act on which segment of the nephron?
- A. Proximal convoluted tubule
- B. Descending limb of the loop of Henle
- C. Ascending limb of the loop of Henle
- D. Distal convoluted tubule
Answer: D. Distal convoluted tubule
27. The core chemical structure of thiazide diuretics is the:
- A. Pyridine ring
- B. 1,2,4-Benzothiadiazine-1,1-dioxide nucleus
- C. Phenothiazine nucleus
- D. Xanthine nucleus
Answer: B. 1,2,4-Benzothiadiazine-1,1-dioxide nucleus
28. Thiazide diuretics inhibit which transporter in the distal convoluted tubule?
- A. Na+/K+/2Cl- symporter
- B. Na+/Cl- symporter (NCC)
- C. Na+/H+ antiporter
- D. Epithelial sodium channel (ENaC)
Answer: B. Na+/Cl- symporter (NCC)
29. For diuretic activity in the thiazide class, which group is essential at position 7 of the benzothiadiazine nucleus?
- A. An amino group (-NH2)
- B. A methyl group (-CH3)
- C. A sulfamoyl group (-SO2NH2)
- D. A carboxyl group (-COOH)
Answer: C. A sulfamoyl group (-SO2NH2)
30. Saturation or removal of the double bond between N2 and C3 in the thiazide nucleus leads to:
- A. Increased diuretic potency (e.g., hydrochlorothiazide vs. chlorothiazide)
- B. Decreased diuretic potency
- C. Loss of diuretic activity
- D. Change in mechanism of action to a loop diuretic
Answer: A. Increased diuretic potency (e.g., hydrochlorothiazide vs. chlorothiazide)
31. Substitution with a lipophilic group (e.g., alkyl, aralkyl) at which position of the thiazide nucleus generally increases diuretic potency and duration of action?
- A. Position 2 (N2)
- B. Position 3 (C3)
- C. Position 6 (C6)
- D. Position 7 (C7)
Answer: B. Position 3 (C3)
32. Chlorthalidone is considered a “thiazide-like” diuretic. How does its core structure differ from true thiazides?
- A. It contains a furan ring instead of a thiadiazine ring.
- B. It has a phthalimidine moiety instead of the benzothiadiazine dioxide ring, but retains a critical sulfamoyl group.
- C. It lacks the sulfonamide group at position 7.
- D. It is a prodrug that is converted to hydrochlorothiazide.
Answer: B. It has a phthalimidine moiety instead of the benzothiadiazine dioxide ring, but retains a critical sulfamoyl group.
33. Indapamide is another thiazide-like diuretic. A key structural feature that distinguishes it is:
- A. The presence of a sulfhydryl group
- B. An indoline ring and a methylindoline substituent on the sulfamoyl nitrogen
- C. Its derivation from a steroid backbone
- D. A pteridine ring system
Answer: B. An indoline ring and a methylindoline substituent on the sulfamoyl nitrogen
34. The electron-withdrawing group typically found at position 6 (e.g., -Cl, -CF3) in thiazide diuretics is important for:
- A. Increasing water solubility
- B. Enhancing the acidity of the sulfonamide group at N2, contributing to activity
- C. Facilitating metabolism
- D. Binding to plasma proteins
Answer: B. Enhancing the acidity of the sulfonamide group at N2, contributing to activity
35. Which of the following is a common metabolic pathway for some thiazide diuretics?
- A. Extensive oxidation of the aromatic ring
- B. N-dealkylation at position 2
- C. Hydrolysis of the sulfamoyl group
- D. Many are excreted largely unchanged or as glucuronides.
Answer: D. Many are excreted largely unchanged or as glucuronides.
36. A major side effect associated with the medicinal chemistry and mechanism of thiazide diuretics is:
- A. Hypercalcemia
- B. Hypokalemia
- C. Hypermagnesemia
- D. Hypernatremia
Answer: B. Hypokalemia
37. Hydrochlorothiazide has a pKa related to the N2-sulfonamide hydrogen of around 7-8. At physiological pH, it will exist:
- A. Mostly in its ionized (anionic) form
- B. Mostly in its non-ionized form
- C. As a zwitterion
- D. Approximately equally in ionized and non-ionized forms
Answer: D. Approximately equally in ionized and non-ionized forms (as it’s close to physiological pH, significant portions of both exist)
38. Replacement of the chlorine atom at C6 of hydrochlorothiazide with a trifluoromethyl group (-CF3) generally results in:
- A. Decreased potency
- B. Increased lipid solubility and potentially longer duration of action (e.g., bendroflumethiazide)
- C. Loss of diuretic activity
- D. A change in the site of action to the proximal tubule
Answer: B. Increased lipid solubility and potentially longer duration of action (e.g., bendroflumethiazide)
39. Metolazone, a quinazolinone derivative, is a thiazide-like diuretic that is known to be effective even in patients with:
- A. Severe hyperkalemia
- B. Advanced renal impairment (low GFR)
- C. Severe hyponatremia
- D. Sulfa allergy (it is also a sulfonamide derivative)
Answer: B. Advanced renal impairment (low GFR)
40. The primary mechanism by which thiazide diuretics lower blood pressure in the long term is thought to involve:
- A. Only initial volume depletion
- B. Reduction in peripheral vascular resistance
- C. Direct beta-adrenergic blockade
- D. Inhibition of the renin-angiotensin system
Answer: B. Reduction in peripheral vascular resistance
41. The acidity of the N2 proton in the thiazide ring system is crucial for its interaction with the Na+/Cl- symporter. This acidity is enhanced by:
- A. Electron-donating groups at C6
- B. The presence of the 1,1-dioxide moiety and the C7-sulfamoyl group
- C. Saturation of the C3=N2 double bond
- D. Alkylation at the C3 position
Answer: B. The presence of the 1,1-dioxide moiety and the C7-sulfamoyl group
42. Polythiazide has a longer duration of action than hydrochlorothiazide. This can be attributed to:
- A. Its higher water solubility
- B. More extensive protein binding and lipid solubility due to its C3 substituent (CH2SCH2CF3)
- C. Rapid conversion to an active metabolite
- D. Resistance to metabolism
Answer: B. More extensive protein binding and lipid solubility due to its C3 substituent (CH2SCH2CF3)
43. A potential drug interaction with thiazide diuretics involves lithium, where thiazides can:
- A. Increase lithium excretion, reducing its efficacy
- B. Decrease lithium excretion, increasing risk of toxicity
- C. Directly antagonize lithium’s therapeutic effect
- D. Accelerate the metabolism of lithium
Answer: B. Decrease lithium excretion, increasing risk of toxicity
44. Which functional group in the general thiazide structure is responsible for the potential for hypersensitivity reactions (sulfa allergy)?
- A. The C3 substituent
- B. The N2 nitrogen
- C. The C7-sulfamoyl group (-SO2NH2)
- D. The C6 chloro or trifluoromethyl group
Answer: C. The C7-sulfamoyl group (-SO2NH2)
45. The term “high-ceiling diuretics” refers to loop diuretics, not thiazides. Thiazide diuretics are considered to have a:
- A. Very high efficacy, similar to loop diuretics
- B. Moderate efficacy or “low ceiling”
- C. Variable efficacy depending on the C6 substituent
- D. Efficacy that increases indefinitely with dose
Answer: B. Moderate efficacy or “low ceiling”
46. If the sulfamoyl group at C7 of a thiazide diuretic is replaced by a carboxyl or methyl group, the diuretic activity is typically:
- A. Significantly increased
- B. Largely retained
- C. Greatly diminished or lost
- D. Converted to carbonic anhydrase inhibitory activity
Answer: C. Greatly diminished or lost
47. For thiazide-like diuretics such as chlorthalidone and indapamide, the diuretic effect is still dependent on which key pharmacophoric element also found in true thiazides?
- A. A condensed thiadiazine ring
- B. An unsubstituted sulfamoyl group (-SO2NH2) or a closely related variant
- C. A lipophilic group at a position equivalent to C3
- D. An electron-withdrawing group at a position equivalent to C6
Answer: B. An unsubstituted sulfamoyl group (-SO2NH2) or a closely related variant
48. The onset of action for most orally administered thiazide diuretics is typically within:
- A. 5-10 minutes
- B. 1-2 hours
- C. 6-8 hours
- D. 24 hours
Answer: B. 1-2 hours
49. Which of the following statements best describes the SAR at position N2 of the thiazide ring?
- A. Substitution with a large alkyl group enhances activity.
- B. It must be unsubstituted (N-H) for optimal activity.
- C. Acylation or alkylation can sometimes be done to create prodrugs or modify duration.
- D. It is typically a quaternary nitrogen.
Answer: B. It must be unsubstituted (N-H) for optimal activity. (Though some modifications exist, the unsubstituted N-H is classic for acidity needed) Correction: Alkylation at N2 generally reduces or abolishes activity. The N2-H is important for the acidic nature. So B is the better choice.
50. The long duration of action of chlorthalidone (a thiazide-like diuretic) is partly due to its:
- A. Rapid metabolism to an active metabolite with a long half-life
- B. Extensive binding to carbonic anhydrase in red blood cells
- C. Enterohepatic recirculation
- D. Very high water solubility leading to slow renal clearance
Answer: B. Extensive binding to carbonic anhydrase in red blood cells

I am a Registered Pharmacist under the Pharmacy Act, 1948, and the founder of PharmacyFreak.com. I hold a Bachelor of Pharmacy degree from Rungta College of Pharmaceutical Science and Research. With a strong academic foundation and practical knowledge, I am committed to providing accurate, easy-to-understand content to support pharmacy students and professionals. My aim is to make complex pharmaceutical concepts accessible and useful for real-world application.
Mail- Sachin@pharmacyfreak.com