Electron transport chain (ETC) – mechanism MCQs With Answer
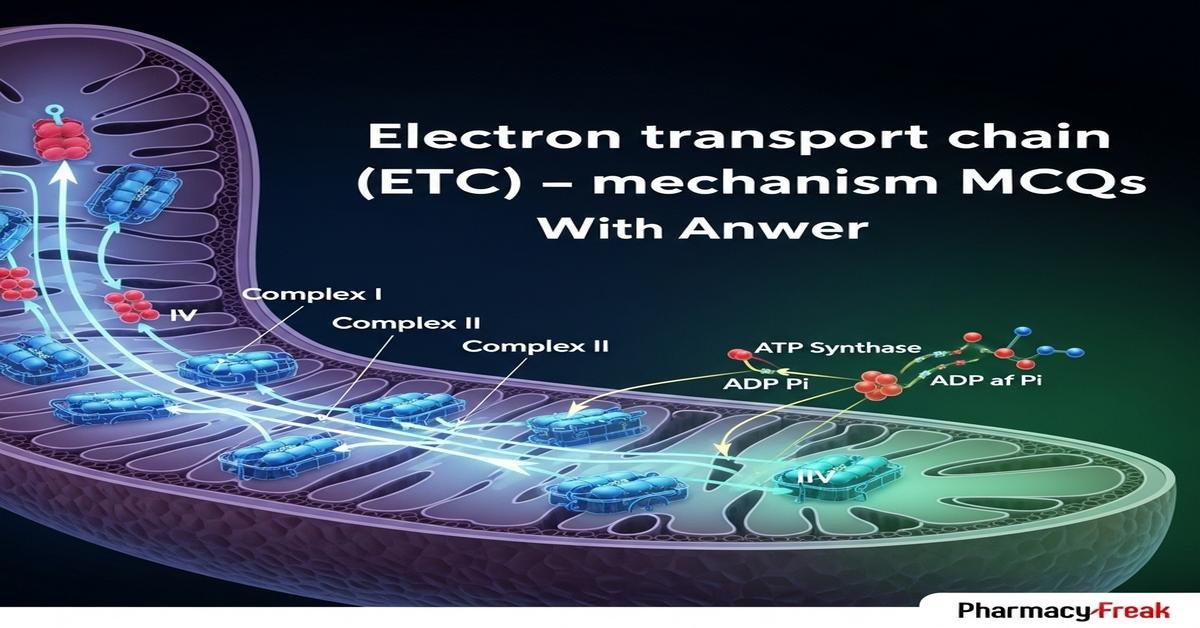
The electron transport chain (ETC) is a central concept in bioenergetics and pharmacology for B.Pharm students, explaining how NADH and FADH2 donate electrons through complexes I–IV, driving proton pumping across the inner mitochondrial membrane to generate a proton motive force that powers ATP synthesis via ATP synthase. Understanding ETC components (ubiquinone, cytochromes, iron–sulfur centers), inhibitors (rotenone, antimycin A, cyanide), uncouplers (DNP), and clinical implications is essential for drug action and toxicity. This set of focused, mechanism-based MCQs will deepen conceptual knowledge and exam readiness. Now let’s test your knowledge with 50 MCQs on this topic.
Q1. Which cellular location houses the electron transport chain (ETC) in eukaryotic cells?
- Outer mitochondrial membrane
- Inner mitochondrial membrane
- Matrix of mitochondria
- Intermembrane space of mitochondria
Correct Answer: Inner mitochondrial membrane
Q2. Which complex of the ETC is primarily responsible for oxidizing NADH?
- Complex II (Succinate dehydrogenase)
- Complex I (NADH:ubiquinone oxidoreductase)
- Complex III (Cytochrome bc1 complex)
- Complex IV (Cytochrome c oxidase)
Correct Answer: Complex I (NADH:ubiquinone oxidoreductase)
Q3. Ubiquinone (coenzyme Q) functions in the ETC as:
- A mobile electron carrier between Complexes I/II and III
- A proton pump embedded in Complex IV
- The final electron acceptor of the chain
- An ATP-binding protein of F1-ATP synthase
Correct Answer: A mobile electron carrier between Complexes I/II and III
Q4. Which component accepts electrons from cytochrome c?
- Complex I
- Complex II
- Complex III
- Complex IV
Correct Answer: Complex IV
Q5. Which of the following is a direct inhibitor of Complex IV (cytochrome c oxidase)?
- Rotenone
- Antimycin A
- Cyanide (CN−)
- Oligomycin
Correct Answer: Cyanide (CN−)
Q6. Which complex of the ETC is also an enzyme in the TCA cycle?
- Complex I
- Complex II (Succinate dehydrogenase)
- Complex III
- Complex IV
Correct Answer: Complex II (Succinate dehydrogenase)
Q7. The chemiosmotic theory proposed by Peter Mitchell states that ATP synthesis is driven primarily by:
- Substrate-level phosphorylation
- Proton motive force across the inner mitochondrial membrane
- Direct heat generation in mitochondria
- Diffusion of ADP into the matrix
Correct Answer: Proton motive force across the inner mitochondrial membrane
Q8. Which subunit of ATP synthase forms the proton channel through the membrane?
- F1 catalytic head
- c-ring within F0
- Beta subunit of F1
- Gamma stalk of F1
Correct Answer: c-ring within F0
Q9. Typical cellular P/O ratio for one NADH leading to ATP synthesis is approximately:
- 1.0 ATP per NADH
- 1.5 ATP per NADH
- 2.5 ATP per NADH
- 4.0 ATP per NADH
Correct Answer: 2.5 ATP per NADH
Q10. Which inhibitor blocks electron transfer at Complex I?
- Antimycin A
- Rotenone
- Cyanide
- Oligomycin
Correct Answer: Rotenone
Q11. Which molecule shuttles electrons between Complex III and Complex IV?
- Ubiquinone (CoQ)
- Cytochrome c
- FMN
- Iron-sulfur protein
Correct Answer: Cytochrome c
Q12. Antimycin A inhibits electron transfer by binding to which complex?
- Complex I
- Complex II
- Complex III
- Complex IV
Correct Answer: Complex III
Q13. What is the primary proton-pumping stoichiometry (protons translocated per 2 electrons) for Complex I?
- 2 protons
- 4 protons
- 6 protons
- 0 protons
Correct Answer: 4 protons
Q14. Which of the following is classified as an uncoupler of oxidative phosphorylation?
- Oligomycin
- 2,4-Dinitrophenol (DNP)
- Rotenone
- Antimycin A
Correct Answer: 2,4-Dinitrophenol (DNP)
Q15. Oligomycin inhibits ATP synthesis by binding to which part of ATP synthase?
- F1 catalytic head
- F0 proton channel
- ADP/ATP translocase
- citrate synthase
Correct Answer: F0 proton channel
Q16. Which redox center contains heme and transfers a single electron within Complex III?
- Fe-S Rieske center
- Cytochrome b heme
- Cytochrome c heme
- FMN
Correct Answer: Fe-S Rieske center
Q17. Which of these statements about reactive oxygen species (ROS) in mitochondria is correct?
- ROS are only produced when ETC is completely blocked
- Superoxide can be generated by electron leakage from complexes I and III
- Mitochondria are incapable of ROS detoxification
- ROS production decreases with high proton motive force
Correct Answer: Superoxide can be generated by electron leakage from complexes I and III
Q18. Which prosthetic group in Complex I initially accepts electrons from NADH?
- Heme a3
- FMN (flavin mononucleotide)
- Ubiquinone
- Cytochrome c
Correct Answer: FMN (flavin mononucleotide)
Q19. The term “proton motive force” consists of which two components?
- Membrane potential (Δψ) and proton concentration difference (ΔpH)
- ATP concentration and ADP concentration
- Oxygen tension and NADH level
- Membrane fluidity and temperature
Correct Answer: Membrane potential (Δψ) and proton concentration difference (ΔpH)
Q20. Which drug blocks electron flow by binding to the Qo site of Complex III?
- Rotenone
- Antimycin A
- Myxothiazol
- Cyanide
Correct Answer: Myxothiazol
Q21. FADH2 contributes electrons to the ETC by donating to:
- Complex I
- Complex II
- Complex III
- Complex IV
Correct Answer: Complex II
Q22. Which of the following best describes the role of iron–sulfur (Fe–S) clusters in ETC?
- They pump protons across the membrane
- They act as mobile lipid carriers
- They transfer single electrons between protein centers
- They hydrolyze ATP
Correct Answer: They transfer single electrons between protein centers
Q23. Which complex reduces molecular oxygen to water?
- Complex II
- Complex III
- Complex IV (cytochrome c oxidase)
- ATP synthase
Correct Answer: Complex IV (cytochrome c oxidase)
Q24. Which molecule is the final electron acceptor in aerobic respiration?
- NAD+
- Fumarate
- Oxygen (O2)
- Pyruvate
Correct Answer: Oxygen (O2)
Q25. Which of the following inhibits ATP synthase directly?
- 2,4-Dinitrophenol
- Oligomycin
- Cyanide
- Rotenone
Correct Answer: Oligomycin
Q26. Which statement about Complex II is true?
- Complex II pumps protons across the membrane
- Complex II transfers electrons from succinate to ubiquinone
- Complex II reduces cytochrome c directly
- Complex II contains copper centers
Correct Answer: Complex II transfers electrons from succinate to ubiquinone
Q27. Which carrier is lipid-soluble and diffuses within the membrane?
- Cytochrome c
- Ubiquinone (CoQ)
- FMN
- Copper A center
Correct Answer: Ubiquinone (CoQ)
Q28. If an uncoupler collapses the proton gradient, which effect is expected?
- ATP synthesis increases while oxygen consumption decreases
- ATP synthesis decreases while oxygen consumption increases
- Both ATP synthesis and oxygen consumption decrease
- No change in ATP synthesis or oxygen consumption
Correct Answer: ATP synthesis decreases while oxygen consumption increases
Q29. Which enzyme catalyzes the reversible conversion of ADP and inorganic phosphate to ATP using the proton gradient?
- ATP synthase (F1F0-ATPase)
- NADH dehydrogenase
- Citrate synthase
- Succinate dehydrogenase
Correct Answer: ATP synthase (F1F0-ATPase)
Q30. Inhibition of Complex III would most directly cause accumulation of which reduced carrier?
- Ubiquinol (QH2)
- Oxidized ubiquinone (Q)
- Oxidized cytochrome c
- Oxygen
Correct Answer: Ubiquinol (QH2)
Q31. Which proton gradient component usually contributes the most to the proton motive force in mitochondria?
- ΔpH (pH gradient)
- Δψ (membrane potential)
- ATP gradient
- Calcium gradient
Correct Answer: Δψ (membrane potential)
Q32. The inhibitory effect of cyanide leads to which immediate change?
- Block of electron flow at Complex I
- Inhibition of Complex IV and rapid cessation of oxygen consumption
- Uncoupling of oxidative phosphorylation
- Activation of ATP synthase
Correct Answer: Inhibition of Complex IV and rapid cessation of oxygen consumption
Q33. Which of the following best explains how ATP synthase synthesizes ATP?
- Electron transfer directly phosphorylates ADP
- Rotation of the central stalk driven by proton flow induces conformational changes in F1 catalytic sites
- Substrate-level phosphorylation in the matrix provides ATP for export
- ATP is synthesized by mechanical compression of ADP and Pi
Correct Answer: Rotation of the central stalk driven by proton flow induces conformational changes in F1 catalytic sites
Q34. Which clinical toxin acts as an uncoupler and was historically used for weight loss with dangerous side effects?
- Rotenone
- Cyanide
- 2,4-Dinitrophenol (DNP)
- Oligomycin
Correct Answer: 2,4-Dinitrophenol (DNP)
Q35. The glycerol-3-phosphate shuttle transfers reducing equivalents from cytosolic NADH to which carrier in mitochondria?
- Complex I directly
- Ubiquinone (via mitochondrial glycerol-3-phosphate dehydrogenase)
- Cytochrome c
- Complex IV
Correct Answer: Ubiquinone (via mitochondrial glycerol-3-phosphate dehydrogenase)
Q36. Which statement about ATP/ADP translocase (ANT) is correct?
- ANT imports ATP into the mitochondrial matrix and exports ADP to the cytosol
- ANT exports ATP to the cytosol and imports ADP into the matrix
- ANT synthesizes ATP from ADP and Pi
- ANT pumps protons across the inner membrane
Correct Answer: ANT exports ATP to the cytosol and imports ADP into the matrix
Q37. Which amino acid residue or prosthetic group is essential in cytochrome a3 for oxygen reduction?
- Iron in heme a3
- FMN
- Ubiquinone headgroup
- Rieske Fe-S center
Correct Answer: Iron in heme a3
Q38. Which change would decrease the proton motive force across the inner mitochondrial membrane?
- Increased activity of Complexes I–IV pumping protons out
- Addition of an uncoupler that permits proton backflow
- Enhanced ATP synthase activity using the gradient
- Increased membrane impermeability to protons
Correct Answer: Addition of an uncoupler that permits proton backflow
Q39. Which of the following is NOT a typical component of Complex IV?
- Heme a
- Copper centers (CuA and CuB)
- FMN
- Heme a3
Correct Answer: FMN
Q40. Reverse electron transport (RET) is most likely when which condition is present?
- High NADH/NAD+ ratio with low proton motive force
- High proton motive force and highly reduced ubiquinone pool
- Complete absence of oxygen
- Maximum ATP demand and low membrane potential
Correct Answer: High proton motive force and highly reduced ubiquinone pool
Q41. What effect does thermogenin (uncoupling protein 1, UCP1) have in brown adipose tissue?
- It increases ATP synthesis efficiency
- It dissipates proton gradient to generate heat
- It inhibits Complex I activity
- It binds oxygen to form water
Correct Answer: It dissipates proton gradient to generate heat
Q42. Which experimental observation supports Mitchell’s chemiosmotic theory?
- ATP can be synthesized without a pH gradient or membrane potential
- Artificial proton gradients across lipid vesicles drive ATP synthesis by reconstituted ATP synthase
- Electron transport occurs only when ATP is abundant
- Oxygen consumption decreases when protons are pumped out
Correct Answer: Artificial proton gradients across lipid vesicles drive ATP synthesis by reconstituted ATP synthase
Q43. Which vitamin-derived cofactor is directly involved in Complex I electron transfer?
- Thiamine pyrophosphate (TPP)
- Flavin mononucleotide (FMN) derived from riboflavin
- Nicotinic acid (niacin) as free NAD+
- Ascorbic acid (vitamin C)
Correct Answer: Flavin mononucleotide (FMN) derived from riboflavin
Q44. Which experimental inhibitor would you use to block ATP synthase and thereby measure proton leak?
- Rotenone
- Oligomycin
- Antimycin A
- Cyanide
Correct Answer: Oligomycin
Q45. How many protons are typically required to synthesize one ATP molecule in mammalian mitochondria (approximate modern estimate)?
- ~1 proton
- ~3 protons
- ~8 protons
- ~12 protons
Correct Answer: ~3 protons
Q46. Which of the following is a consequence of ETC inhibition leading to decreased ATP in neurons?
- Enhanced neurotransmitter release due to excess energy
- Failure of ion pumps, membrane depolarization and neuronal dysfunction
- Increased protein synthesis
- Decreased cytosolic calcium levels permanently
Correct Answer: Failure of ion pumps, membrane depolarization and neuronal dysfunction
Q47. During fatty acid β-oxidation, FADH2 produced transfers electrons into the ETC at which entry point?
- Directly to Complex I
- To ubiquinone via electron-transferring flavoprotein (ETF) and ETF-ubiquinone oxidoreductase
- To cytochrome c
- Directly to Complex IV
Correct Answer: To ubiquinone via electron-transferring flavoprotein (ETF) and ETF-ubiquinone oxidoreductase
Q48. Which molecular property of ubiquinone allows it to shuttle electrons within the membrane?
- Its water-soluble quinone head only
- Its hydrophobic isoprenoid tail enabling mobility in the lipid bilayer
- Its covalent attachment to Complex I
- Its localization to the matrix
Correct Answer: Its hydrophobic isoprenoid tail enabling mobility in the lipid bilayer
Q49. Which pathology is most directly linked to defects in mitochondrial ETC proteins?
- Cystic fibrosis
- Mitochondrial myopathies and neurodegenerative diseases
- Type I diabetes due to insulin deficiency
- Hemophilia due to clotting factor deficiency
Correct Answer: Mitochondrial myopathies and neurodegenerative diseases
Q50. Which measurement gives a direct assessment of mitochondrial coupling efficiency?
- Rate of glycolysis in the cytosol
- Oxygen consumption in the presence and absence of ADP (respiratory control ratio)
- Total cellular protein content
- Membrane cholesterol content
Correct Answer: Oxygen consumption in the presence and absence of ADP (respiratory control ratio)

I am a Registered Pharmacist under the Pharmacy Act, 1948, and the founder of PharmacyFreak.com. I hold a Bachelor of Pharmacy degree from Rungta College of Pharmaceutical Science and Research. With a strong academic foundation and practical knowledge, I am committed to providing accurate, easy-to-understand content to support pharmacy students and professionals. My aim is to make complex pharmaceutical concepts accessible and useful for real-world application.
Mail- Sachin@pharmacyfreak.com