Introduction
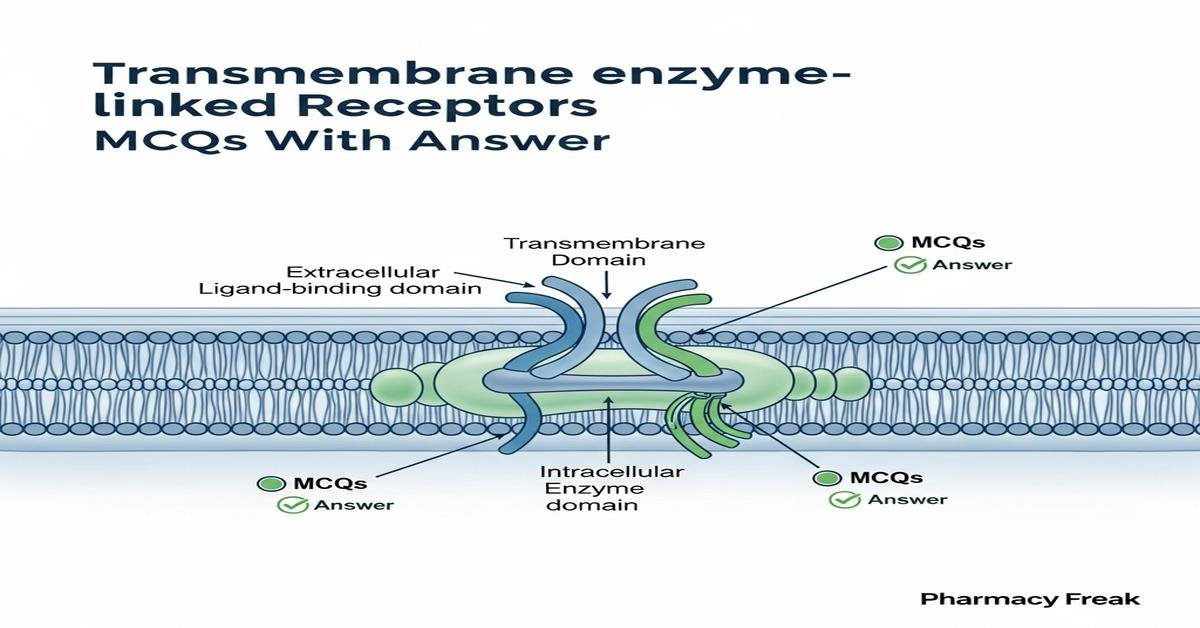
Transmembrane enzyme-linked receptors are integral membrane proteins that couple extracellular ligand binding to intrinsic or associated enzymatic activity, initiating signal transduction cascades. Key families include receptor tyrosine kinases (RTKs), receptor serine/threonine kinases and particulate guanylyl cyclases. Understanding ligand-induced dimerization, autophosphorylation, downstream pathways (MAPK, PI3K–Akt), and pharmacological modulation is essential for B. Pharm students studying pharmacology and drug design. Clinical relevance spans cancer therapy, metabolic disease and targeted kinase inhibitors (e.g., imatinib, gefitinib). This set of MCQs emphasizes mechanisms, receptor domains, signaling outcomes, assays and therapeutic strategies to deepen concept mastery. Now let’s test your knowledge with 30 MCQs on this topic.
Q1. Which feature best defines a transmembrane enzyme-linked receptor?
- An intracellular receptor that binds steroid hormones
- A membrane receptor with intrinsic or associated enzymatic activity activated by ligand binding
- A G protein-coupled receptor that activates adenylate cyclase
- A channel that allows passive ion flow upon voltage change
Correct Answer: A membrane receptor with intrinsic or associated enzymatic activity activated by ligand binding
Q2. Which of the following is a classic example of a receptor tyrosine kinase (RTK)?
- Beta-adrenergic receptor
- Epidermal growth factor receptor (EGFR)
- Ligand-gated ion channel
Correct Answer: Epidermal growth factor receptor (EGFR)
Q3. What immediate event typically follows ligand binding to many RTKs?
- Receptor internalization without phosphorylation
- Dimerization and trans-autophosphorylation of tyrosine residues
- Cleavage of intracellular domain by proteases
- G protein activation and cAMP increase
Correct Answer: Dimerization and trans-autophosphorylation of tyrosine residues
Q4. The insulin receptor is classified as which type of enzyme-linked receptor?
- Receptor guanylyl cyclase
- Receptor serine/threonine kinase
- Receptor tyrosine kinase
- Toll-like receptor
Correct Answer: Receptor tyrosine kinase
Q5. Which downstream signaling pathway is commonly activated by RTKs and is central to cell proliferation?
- MAPK/ERK pathway
- cGMP-dependent kinase pathway
- Glucocorticoid receptor pathway
- Voltage-gated calcium pathway
Correct Answer: MAPK/ERK pathway
Q6. Which domain is essential for the catalytic activity of RTKs?
- Extracellular ligand-binding domain
- Transmembrane alpha-helix
- Intracellular tyrosine kinase domain
- Pleckstrin homology domain in the extracellular region
Correct Answer: Intracellular tyrosine kinase domain
Q7. Receptor guanylyl cyclases produce which second messenger upon activation?
- cAMP
- IP3
- cGMP
- Diacylglycerol (DAG)
Correct Answer: cGMP
Q8. Which pharmacological class directly inhibits the kinase activity of RTKs?
- Monoclonal antibodies against nuclear receptors
- Small-molecule tyrosine kinase inhibitors (TKIs)
- Beta-blockers
- ACE inhibitors
Correct Answer: Small-molecule tyrosine kinase inhibitors (TKIs)
Q9. Imatinib is an example of a drug that targets which molecular mechanism?
- DNA alkylation in tumor cells
- ATP-binding site of a tyrosine kinase
- Extracellular ligand sequestration
- G protein inhibition
Correct Answer: ATP-binding site of a tyrosine kinase
Q10. Which mutation in RTKs commonly leads to constitutive activation and oncogenesis?
- Deletion of extracellular ligand-binding domain resulting in ligand-independent dimerization
- Mutation that prevents membrane insertion of the receptor
- Loss of the cytoplasmic domain without kinase disruption
- Point mutation in the extracellular glycosylation site only
Correct Answer: Deletion of extracellular ligand-binding domain resulting in ligand-independent dimerization
Q11. How do monoclonal antibodies against RTKs typically exert therapeutic effects?
- By directly inhibiting intracellular ATP binding
- By blocking ligand binding or inducing receptor internalization
- By degrading downstream MAPK proteins
- By activating G proteins
Correct Answer: By blocking ligand binding or inducing receptor internalization
Q12. Which assay is commonly used to measure autophosphorylation of RTKs in research?
- ELISA for phosphorylated tyrosine residues
- Patch-clamp electrophysiology
- Chromatography for small molecules
- Gram staining
Correct Answer: ELISA for phosphorylated tyrosine residues
Q13. The juxtamembrane region of many RTKs regulates what function?
- Transcription factor binding in the nucleus
- Kinase activation and receptor stability
- Mitochondrial membrane potential
- Production of cyclic AMP
Correct Answer: Kinase activation and receptor stability
Q14. Which of the following best describes “transphosphorylation”?
- A receptor phosphorylates a downstream cytosolic protein without receptor modification
- Two receptor monomers phosphorylate each other on tyrosine residues
- Autophosphorylation of a single receptor molecule on serine residues only
- Phosphorylation of receptor by an unrelated cytosolic kinase exclusively
Correct Answer: Two receptor monomers phosphorylate each other on tyrosine residues
Q15. Which downstream effector binds phosphotyrosine residues on activated RTKs via SH2 domains?
- Phospholipase Cγ (PLCγ)
- DNA polymerase
- Glycogen synthase
- Lipid A
Correct Answer: Phospholipase Cγ (PLCγ)
Q16. Resistance to TKIs in cancer often arises by which mechanism?
- Increased endocytosis of the drug
- Secondary mutations in the kinase domain reducing drug binding
- Complete loss of the target receptor gene
- Enhanced extracellular ligand degradation
Correct Answer: Secondary mutations in the kinase domain reducing drug binding
Q17. Which type of enzyme-linked receptor mediates signaling via SMAD proteins?
- Receptor tyrosine kinases
- Receptor serine/threonine kinases (e.g., TGF-β receptors)
- Ionotropic glutamate receptors
- Receptor tyrosine phosphatases
Correct Answer: Receptor serine/threonine kinases (e.g., TGF-β receptors)
Q18. Which therapeutic strategy targets ligand availability rather than receptor activity?
- Small-molecule kinase inhibitors
- Ligand-neutralizing antibodies or soluble decoy receptors
- Proteasome inhibitors
- Ion channel blockers
Correct Answer: Ligand-neutralizing antibodies or soluble decoy receptors
Q19. Which statement about receptor endocytosis after ligand binding is correct?
- Endocytosis always increases receptor signaling indefinitely
- Endocytosis can terminate signaling or route receptors to endosomes for signaling modulation
- Endocytosis only occurs for GPCRs, not enzyme-linked receptors
- Endocytosis is independent of ubiquitination status
Correct Answer: Endocytosis can terminate signaling or route receptors to endosomes for signaling modulation
Q20. The PI3K–Akt pathway activated by some RTKs primarily promotes which cellular outcome?
- Cell apoptosis and degradation
- Cell survival, growth and metabolism
- Immediate ion flux through the membrane
- Chromatin condensation during mitosis
Correct Answer: Cell survival, growth and metabolism
Q21. A tyrosine kinase receptor inhibitor that competes with ATP will most directly affect which process?
- Ligand binding to the extracellular domain
- Phosphorylation of tyrosine residues on substrates
- Receptor gene transcription
- Endosomal acidification
Correct Answer: Phosphorylation of tyrosine residues on substrates
Q22. Which experimental technique helps identify phosphorylation sites on RTKs?
- Mass spectrometry of tryptic peptides
- Southern blotting
- Light microscopy without labeling
- pH titration curve analysis
Correct Answer: Mass spectrometry of tryptic peptides
Q23. In receptor serine/threonine kinases, which residue type is phosphorylated to transmit signal?
- Tyrosine
- Serine and threonine
- Lysine
- Histidine
Correct Answer: Serine and threonine
Q24. The extracellular domain of enzyme-linked receptors is primarily responsible for what?
- ATP binding and hydrolysis
- Ligand recognition and specificity
- Direct phosphorylation of cytosolic proteins
- Generating second messengers inside the nucleus
Correct Answer: Ligand recognition and specificity
Q25. Which of the following is a potential adverse effect of systemic RTK inhibition?
- Improved wound healing universally
- Hypertension, skin rash or impaired wound healing due to off-target effects
- Immediate bacterial infection due to kinase inhibition
- Permanent enhancement of fertility
Correct Answer: Hypertension, skin rash or impaired wound healing due to off-target effects
Q26. Which adaptor protein commonly links RTK activation to Ras activation?
- Adaptor protein Grb2
- Cytochrome c
- Myosin light chain
- RNA polymerase II
Correct Answer: Adaptor protein Grb2
Q27. Which statement describes receptor tyrosine phosphatases (RPTPs)?
- They are soluble enzymes with no membrane anchor
- They are transmembrane proteins that remove phosphate groups from tyrosine residues
- They exclusively phosphorylate serine residues
- They always act as oncogenes when overexpressed
Correct Answer: They are transmembrane proteins that remove phosphate groups from tyrosine residues
Q28. Which clinical test might assess RTK pathway activation in a tumor biopsy?
- Immunohistochemistry for phosphorylated ERK or phosphorylated receptor
- Urine dipstick test for glucose
- Electrocardiogram (ECG)
- Complete blood count only
Correct Answer: Immunohistochemistry for phosphorylated ERK or phosphorylated receptor
Q29. A soluble decoy receptor for VEGF works by which mechanism?
- Enhancing VEGF binding to VEGFR on endothelial cells
- Sequestering VEGF ligand and preventing receptor activation
- Increasing VEGF synthesis in tumor cells
- Blocking nuclear export of VEGF mRNA
Correct Answer: Sequestering VEGF ligand and preventing receptor activation
Q30. Which criterion is most important when designing selective small-molecule inhibitors for RTKs?
- Hydrophobicity only, regardless of binding site
- Specificity for the ATP-binding pocket and unique residues to minimize off-target effects
- A sequence identical to ATP to ensure competition
- Complete inhibition of all kinases in the cell
Correct Answer: Specificity for the ATP-binding pocket and unique residues to minimize off-target effects

I am a Registered Pharmacist under the Pharmacy Act, 1948, and the founder of PharmacyFreak.com. I hold a Bachelor of Pharmacy degree from Rungta College of Pharmaceutical Science and Research. With a strong academic foundation and practical knowledge, I am committed to providing accurate, easy-to-understand content to support pharmacy students and professionals. My aim is to make complex pharmaceutical concepts accessible and useful for real-world application.
Mail- Sachin@pharmacyfreak.com