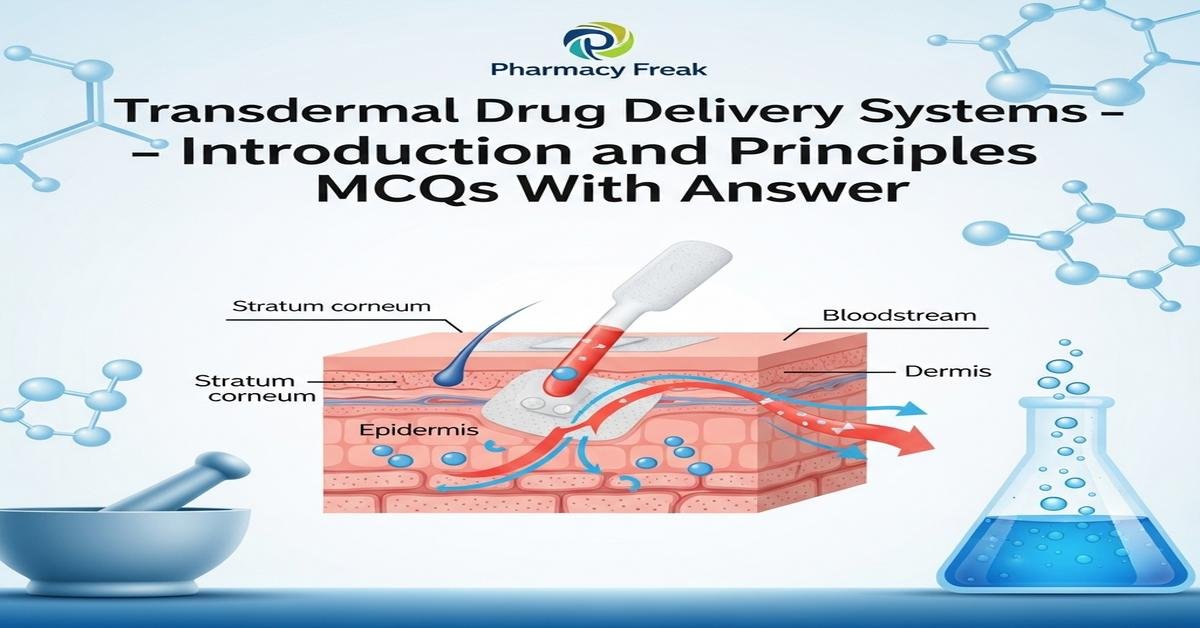
Transdermal drug delivery systems (TDD) provide controlled, non-invasive drug administration through the skin to achieve systemic or local effects. This introduction covers principles like percutaneous absorption, stratum corneum barrier function, Fick’s law of diffusion, partition coefficient, molecular weight limits, and rate-controlling membranes. You will learn formulation types (matrix, reservoir, drug-in-adhesive), role of polymers, permeation enhancers, microneedles, and evaluation tools such as Franz diffusion cells and in vivo pharmacokinetics. Emphasis is on designing patches for optimal bioavailability, safety, adhesion, and steady-state flux. Key terms include patch, permeation enhancers, controlled release, polymers, flux, and skin irritation. Now let’s test your knowledge with 30 MCQs on this topic.
Q1. Which skin layer is the primary barrier to transdermal drug delivery?
- Dermis
- Epidermis
- Stratum corneum
- Hypodermis
Correct Answer: Stratum corneum
Q2. According to typical empirical rules for transdermal delivery, the preferred molecular weight of a drug is:
- Greater than 1000 Da
- Less than 500 Da
- Between 800 and 1200 Da
- Irrelevant to skin permeation
Correct Answer: Less than 500 Da
Q3. Which physicochemical property most strongly favors passive transdermal permeation?
- Very high aqueous solubility only
- Optimal balance of lipophilicity and hydrophilicity (log P ~1–3)
- Extremely high lipophilicity (log P >6)
- High molecular weight and polar surface area
Correct Answer: Optimal balance of lipophilicity and hydrophilicity (log P ~1–3)
Q4. Fick’s first law for steady-state flux through the skin relates flux (J) to:
- Permeability coefficient only
- Drug concentration gradient, permeability coefficient, and membrane thickness
- Temperature and optical density
- Drug ionization state only
Correct Answer: Drug concentration gradient, permeability coefficient, and membrane thickness
Q5. A reservoir transdermal patch differs from a matrix patch by having:
- Drug dispersed in adhesive only
- A separate liquid or gel reservoir with a rate-controlling membrane
- No backing layer
- Only natural rubber adhesives
Correct Answer: A separate liquid or gel reservoir with a rate-controlling membrane
Q6. Which permeability enhancer is commonly used to disrupt stratum corneum lipids?
- Polyethylene glycol 4000
- Oleic acid
- Microcrystalline cellulose
- Sodium chloride
Correct Answer: Oleic acid
Q7. Iontophoresis enhances transdermal delivery primarily by:
- Generating ultrasonic waves to disrupt the skin
- Applying a small electric current to drive charged molecules across the skin
- Heating the patch to increase diffusion
- Encapsulating drugs in liposomes only
Correct Answer: Applying a small electric current to drive charged molecules across the skin
Q8. Microneedles improve transdermal flux by:
- Increasing skin hydration only
- Creating microchannels that bypass the stratum corneum
- Decreasing drug solubility
- Forming a permanent scar barrier
Correct Answer: Creating microchannels that bypass the stratum corneum
Q9. The term “drug-in-adhesive” patch refers to:
- Drug contained in a separate reservoir external to adhesive
- Drug uniformly dispersed directly within the adhesive layer
- Drug applied topically as a cream under the patch
- Patch lacking an adhesive layer
Correct Answer: Drug uniformly dispersed directly within the adhesive layer
Q10. Which polymer is commonly used as an adhesive matrix in transdermal patches?
- Polyisobutylene
- Polylactic acid (PLA) as a rigid implant
- Sodium alginate for enteric coating
- Polyvinyl alcohol as a non-adhesive powder
Correct Answer: Polyisobutylene
Q11. Steady-state transdermal flux is achieved after the:
- Lag time
- Immediate application
- Patch removal
- Drug crystallization
Correct Answer: Lag time
Q12. Which analytical setup is most commonly used for in vitro permeation testing of patches?
- High-performance liquid chromatography only
- Franz diffusion cell
- Gas chromatography-mass spectrometry without membrane
- Petri dish diffusion without receptor phase
Correct Answer: Franz diffusion cell
Q13. Which factor can decrease transdermal drug flux?
- Increasing patch surface area
- Increase in partition coefficient within optimal range
- Increasing membrane thickness
- Applying permeation enhancer
Correct Answer: Increasing membrane thickness
Q14. Reservoir patch failure has led to safety concerns due to:
- Loss of adhesion only
- Dose-dumping when rate-controlling membrane ruptures
- Lack of drug in reservoir from manufacturing
- Incompatibility with polymers only
Correct Answer: Dose-dumping when rate-controlling membrane ruptures
Q15. Which drug property often requires prodrug strategies for effective transdermal delivery?
- Low potency
- High molecular weight or high polarity that limit skin permeation
- High lipid solubility within optimum range
- Already ideal log P and small size
Correct Answer: High molecular weight or high polarity that limit skin permeation
Q16. Occlusion under a transdermal patch primarily increases permeation by:
- Decreasing skin temperature significantly
- Hydrating the stratum corneum, increasing diffusivity
- Changing drug pKa
- Removing epidermal cells mechanically
Correct Answer: Hydrating the stratum corneum, increasing diffusivity
Q17. Which kinetic profile is most desirable for many transdermal therapeutic systems to maintain constant plasma levels?
- First-order burst release only
- Zero-order release
- Random erratic release
- Immediate complete release
Correct Answer: Zero-order release
Q18. In the steady-state permeation equation J = (D·K·C)/h, K represents:
- Diffusion coefficient
- Partition coefficient between skin and vehicle
- Drug concentration in receptor only
- Membrane thickness
Correct Answer: Partition coefficient between skin and vehicle
Q19. Which test is commonly used to assess skin irritation potential of a transdermal patch?
- In vitro cytotoxicity only
- Patch test on human volunteers or animal dermal irritation studies
- Oral toxicity test
- Inhalation challenge
Correct Answer: Patch test on human volunteers or animal dermal irritation studies
Q20. Which excipient class improves skin permeation by fluidizing lipid bilayers in the stratum corneum?
- Humectants only
- Permeation enhancers like ethanol, DMSO, or fatty acids
- Inert fillers like microcrystalline cellulose
- Preservatives only
Correct Answer: Permeation enhancers like ethanol, DMSO, or fatty acids
Q21. A transdermal delivery advantage over oral dosing is:
- Complete elimination of adverse effects
- Avoidance of first-pass hepatic metabolism for many drugs
- Unlimited drug load capacity
- Always faster onset than IV injection
Correct Answer: Avoidance of first-pass hepatic metabolism for many drugs
Q22. Which measurement describes the amount of drug crossing per unit area per unit time?
- Lag time
- Flux (J)
- Permeability coefficient (Kp) only
- Partition ratio
Correct Answer: Flux (J)
Q23. For a hydrophilic drug, which approach can improve skin permeation?
- Decrease drug concentration in the formulation
- Use of iontophoresis or microneedles
- Increasing molecular weight
- Using only hydrophobic polymer matrices without enhancers
Correct Answer: Use of iontophoresis or microneedles
Q24. Which property of a patch backing layer is most important?
- Color only
- Impermeability to drug and protection from external contaminants
- Being edible
- Conductivity for iontophoresis
Correct Answer: Impermeability to drug and protection from external contaminants
Q25. The permeability coefficient (Kp) is calculated as:
- Kp = flux / donor concentration
- Kp = diffusion coefficient × thickness
- Kp = flux × thickness
- Kp = molecular weight / logP
Correct Answer: Kp = flux / donor concentration
Q26. Which manufacturing concern is critical for drug-in-adhesive patches?
- Selecting a non-sticky backing layer
- Ensuring uniform drug distribution within the adhesive
- Maximizing residual solvents in adhesive
- Avoiding use of pressure-sensitive adhesives entirely
Correct Answer: Ensuring uniform drug distribution within the adhesive
Q27. Which evaluation assesses long-term stability of transdermal patches under accelerated conditions?
- Short-term in vivo bioequivalence study only
- Accelerated stability testing at elevated temperature and humidity
- Immediate patch adhesion test only
- Spray-drying analysis
Correct Answer: Accelerated stability testing at elevated temperature and humidity
Q28. Which adverse effect is most often associated with transdermal patches?
- Systemic bleeding in all cases
- Local skin irritation or sensitization
- Permanent loss of skin pigmentation always
- Gastrointestinal ulceration
Correct Answer: Local skin irritation or sensitization
Q29. Which statement about reservoir and matrix patches regarding dose control is true?
- Matrix patches always risk dose-dumping more than reservoir patches
- Reservoir patches can give controlled zero-order release but risk dose-dumping if membrane fails
- Both types cannot control release kinetics
- Neither uses adhesives
Correct Answer: Reservoir patches can give controlled zero-order release but risk dose-dumping if membrane fails
Q30. Which in vivo parameter is most important to compare when evaluating a new transdermal formulation against a reference?
- Color of the patch
- Plasma concentration-time profile and pharmacokinetic parameters (Cmax, AUC)
- Manufacturing cost only
- Pectin content
Correct Answer: Plasma concentration-time profile and pharmacokinetic parameters (Cmax, AUC)

I am a Registered Pharmacist under the Pharmacy Act, 1948, and the founder of PharmacyFreak.com. I hold a Bachelor of Pharmacy degree from Rungta College of Pharmaceutical Science and Research. With a strong academic foundation and practical knowledge, I am committed to providing accurate, easy-to-understand content to support pharmacy students and professionals. My aim is to make complex pharmaceutical concepts accessible and useful for real-world application.
Mail- Sachin@pharmacyfreak.com