Introduction
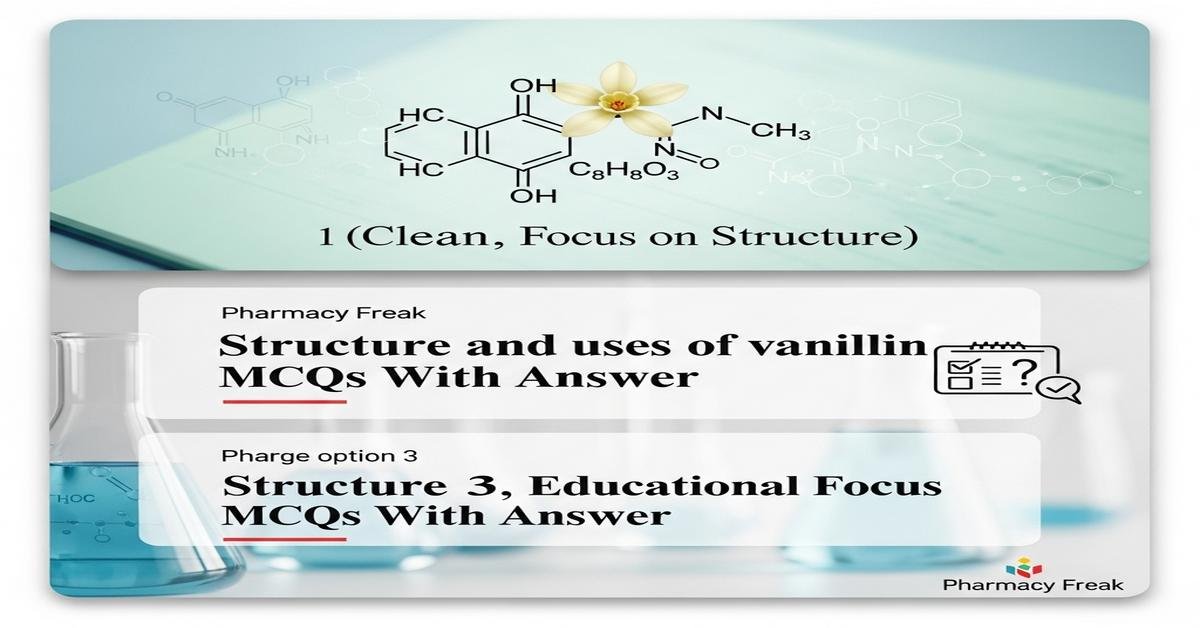
Understanding the structure and uses of vanillin is essential for B.Pharm students studying pharmaceutical excipients, flavoring agents and organic intermediates. Vanillin (4-hydroxy-3-methoxybenzaldehyde) features an aromatic ring with aldehyde, phenolic hydroxyl and methoxy functional groups that determine its reactivity, physical properties and analytical behavior. This topic covers chemical structure, biosynthesis and industrial production routes (lignin, guaiacol, ferulic acid), spectral identification (IR, NMR, UV), chemical transformations (oxidation, reduction, derivatization), pharmaceutical applications (flavoring, masking, antioxidants) and regulatory status. These focused, exam-oriented MCQs will reinforce key concepts, spectral interpretation and formulation roles relevant to pharmacognosy, pharmaceutics and medicinal chemistry. Now let’s test your knowledge with 50 MCQs on this topic.
Q1. What is the IUPAC name of vanillin?
- 3-hydroxy-4-methoxybenzaldehyde
- 4-hydroxy-3-methoxybenzaldehyde
- 3-methoxy-4-hydroxybenzaldehyde
- 4-methoxy-3-hydroxybenzaldehyde
Correct Answer: 4-hydroxy-3-methoxybenzaldehyde
Q2. What is the molecular formula of vanillin?
- C7H6O3
- C8H8O3
- C9H10O3
- C8H6O4
Correct Answer: C8H8O3
Q3. Which functional groups are present in vanillin?
- Carboxylic acid, nitro, methoxy
- Aldehyde, phenolic hydroxyl, methoxy
- Ketone, phenolic hydroxyl, ethoxy
- Aldehyde, amine, methoxy
Correct Answer: Aldehyde, phenolic hydroxyl, methoxy
Q4. Vanillin is commonly produced industrially from which of the following feedstocks?
- Guaiacol and glyoxylic acid
- Salicylic acid and methanol
- Toluene and chlorine
- Phenol and acetone
Correct Answer: Guaiacol and glyoxylic acid
Q5. Which natural polymer is an important source for vanillin through depolymerization?
- Cellulose
- Lignin
- Starch
- Chitin
Correct Answer: Lignin
Q6. The aldehyde proton in vanillin appears in proton NMR at approximately which chemical shift?
- 0.9–1.5 ppm
- 3.6–3.9 ppm
- 7.0–8.0 ppm
- 9.5–10.0 ppm
Correct Answer: 9.5–10.0 ppm
Q7. The methoxy group in vanillin gives a singlet in 1H NMR at around:
- 0.9 ppm
- 2.0 ppm
- 3.7–3.9 ppm
- 7.5 ppm
Correct Answer: 3.7–3.9 ppm
Q8. Which reaction converts vanillin to vanillic acid?
- Reduction with NaBH4
- Oxidation of the aldehyde
- Methylation of the phenol
- Nitration of the aromatic ring
Correct Answer: Oxidation of the aldehyde
Q9. Vanillin gives a positive Tollens’ test because it contains which functional group?
- Phenolic hydroxyl
- Aldehyde
- Methoxy
- Aromatic ring only
Correct Answer: Aldehyde
Q10. Which derivative is known as ethyl vanillin?
- 4-hydroxy-3-ethoxybenzaldehyde
- 3-hydroxy-4-ethoxybenzaldehyde
- 4-ethoxy-3-methoxybenzaldehyde
- 3-ethoxy-4-methoxybenzaldehyde
Correct Answer: 4-hydroxy-3-ethoxybenzaldehyde
Q11. Which spectroscopic technique is most useful for identifying the conjugated aldehyde carbonyl in vanillin?
- Mass spectrometry
- Infrared (IR) spectroscopy
- Polarimetry
- Elemental analysis
Correct Answer: Infrared (IR) spectroscopy
Q12. A strong IR absorption around 1670 cm−1 in vanillin corresponds to:
- Phenolic O–H stretch
- Aromatic C=C stretch
- Conjugated aldehyde C=O stretch
- C–O–C ether stretch
Correct Answer: Conjugated aldehyde C=O stretch
Q13. In pharmaceutical formulations, vanillin is mainly used as a:
- Active pharmaceutical ingredient (API)
- Flavoring and masking agent
- Preservative
- Primary binder
Correct Answer: Flavoring and masking agent
Q14. Which analytical method is commonly used for quantitative determination of vanillin in formulations?
- Thin layer chromatography only
- HPLC with UV detection
- Titration with NaOH
- Direct visual inspection
Correct Answer: HPLC with UV detection
Q15. Vanillin is classified by the FDA as which of the following for food and pharmaceutical uses?
- Prescription drug
- GRAS (Generally Recognized as Safe)
- Controlled substance
- Not permitted for use
Correct Answer: GRAS (Generally Recognized as Safe)
Q16. Which microbial precursor is commonly biotransformed to vanillin in biotechnological processes?
- Benzoic acid
- Ferulic acid
- Citric acid
- Acetic acid
Correct Answer: Ferulic acid
Q17. Reduction of the aldehyde group of vanillin yields which compound?
- Vanillic acid
- Vanillyl alcohol
- Vanillic aldehyde
- Vanillin acetate
Correct Answer: Vanillyl alcohol
Q18. Which property of vanillin contributes to its role as a masking agent in oral syrups?
- High melting point
- Distinct sweet aromatic flavor and odor
- Strong antiseptic activity
- High aqueous solubility
Correct Answer: Distinct sweet aromatic flavor and odor
Q19. Vanillin’s aromatic ring is activated towards electrophilic substitution primarily by which group?
- Methoxy group (–OCH3)
- Aldehyde group (–CHO)
- Nitro group (–NO2)
- Carboxyl group (–COOH)
Correct Answer: Methoxy group (–OCH3)
Q20. Which of the following is a common chemical test to derivatize the aldehyde of vanillin for analytical detection?
- Formation of dinitrophenylhydrazone (DNPH)
- Esterification with methanol
- Diazotization
- Peroxide formation
Correct Answer: Formation of dinitrophenylhydrazone (DNPH)
Q21. Which solvent pair is commonly used for HPLC analysis of vanillin?
- Water–acetonitrile
- Hexane–ethyl acetate
- Carbon tetrachloride–methanol
- Petroleum ether–chloroform
Correct Answer: Water–acetonitrile
Q22. Vanillin shows antioxidant activity primarily because of which feature?
- Aldehyde group donating electrons
- Phenolic hydroxyl capable of hydrogen donation
- Methoxy group forming radicals
- Aromatic ring only
Correct Answer: Phenolic hydroxyl capable of hydrogen donation
Q23. Which of the following best describes vanillin’s solubility?
- Highly soluble in water
- Insoluble in common organic solvents
- Soluble in ethanol and organic solvents but limited in water
- Only soluble in oils
Correct Answer: Soluble in ethanol and organic solvents but limited in water
Q24. The typical melting point range of pure vanillin is approximately:
- 20–25 °C
- 60–62 °C
- 81–83 °C
- 150–155 °C
Correct Answer: 81–83 °C
Q25. Which chromatography method coupled with mass spectrometry is useful for identifying vanillin in complex matrices?
- Gas chromatography–mass spectrometry (GC–MS)
- Paper chromatography
- Size exclusion chromatography
- Ion exchange chromatography
Correct Answer: Gas chromatography–mass spectrometry (GC–MS)
Q26. In medicinal chemistry, vanillin is often used as:
- A starting material for synthesis of more complex molecules
- A controlled drug substance
- A strong acid catalyst
- An isotonic agent
Correct Answer: A starting material for synthesis of more complex molecules
Q27. Which position on the benzene ring of vanillin bears the methoxy substituent?
- Position 1 (aldehyde carbon)
- Position 2 (ortho to aldehyde)
- Position 3 (meta to aldehyde)
- Position 4 (para to aldehyde)
Correct Answer: Position 3 (meta to aldehyde)
Q28. Which reagent selectively reduces the aldehyde group of vanillin to the corresponding alcohol under mild conditions?
- PCC (pyridinium chlorochromate)
- LiAlH4 in ether
- NaBH4 in ethanol
- Chromic acid
Correct Answer: NaBH4 in ethanol
Q29. Vanillin can be converted to Schiff bases by reaction with:
- Primary amines
- Carboxylic acids
- Alcohols
- Alkyl halides
Correct Answer: Primary amines
Q30. Which statement about vanillin’s role in topical pharmaceutical formulations is correct?
- Vanillin acts as a primary antimicrobial preservative
- Vanillin is used mainly as a fragrance or odor masker
- Vanillin is used as a gelling agent
- Vanillin is a common UV absorber in creams
Correct Answer: Vanillin is used mainly as a fragrance or odor masker
Q31. A student analyzing vanillin by UV–Vis spectroscopy would most likely monitor the absorbance near which region?
- 200–220 nm
- 250–300 nm
- 400–450 nm
- 600–700 nm
Correct Answer: 250–300 nm
Q32. Which oxidative biotransformation is industrially used to obtain vanillin from lignin?
- Hydrolytic decarboxylation
- Oxidative depolymerization
- Hydrogenation of aromatic rings
- Reduction of ketones
Correct Answer: Oxidative depolymerization
Q33. Which of the following is a common impurity in crude vanillin obtained from lignin?
- Vanillyl alcohol
- Glucose
- Sucrose
- Benzoic acid
Correct Answer: Vanillyl alcohol
Q34. Vanillin forms which type of protective derivative with 2,4-dinitrophenylhydrazine (DNPH)?
- Hydrazone
- Oxime
- Thioether
- Acetal
Correct Answer: Hydrazone
Q35. Which factor most strongly affects the odor perception of vanillin in formulations?
- pH of the formulation
- Particle size only
- Presence of water only
- Concentration and volatility
Correct Answer: Concentration and volatility
Q36. Which solvent would most likely dissolve vanillin readily for preparative chemistry?
- Diethyl ether
- Water at room temperature
- Ethanol
- n-Hexane
Correct Answer: Ethanol
Q37. Which chemical modification increases the lipophilicity of vanillin for formulation studies?
- Oxidation of the aldehyde to acid
- Acetylation of the phenolic OH
- Hydrogenation of the aromatic ring
- Conversion to the DNPH derivative
Correct Answer: Acetylation of the phenolic OH
Q38. Vanillin exhibits antimicrobial effects primarily due to:
- Its strong basicity
- Phenolic structure disrupting microbial membranes and enzyme systems
- High acidity killing bacteria
- Being a powerful oxidant
Correct Answer: Phenolic structure disrupting microbial membranes and enzyme systems
Q39. In TLC analysis of vanillin, what visualization reagent would reveal the phenolic compound?
- Dragendorff’s reagent
- ninhydrin
- Vanillin-sulfuric acid reagent
- Silver nitrate solution
Correct Answer: Vanillin-sulfuric acid reagent
Q40. Which of the following is NOT a common route to synthesize vanillin?
- From guaiacol
- From eugenol via oxidative cleavage
- Direct extraction from petroleum crude oil
- Bioconversion of ferulic acid
Correct Answer: Direct extraction from petroleum crude oil
Q41. What is a primary environmental advantage of biotechnological production of vanillin from ferulic acid?
- Higher energy consumption
- Lower selectivity
- Use of renewable feedstock and milder conditions
- Greater generation of toxic byproducts
Correct Answer: Use of renewable feedstock and milder conditions
Q42. Which atom count corresponds to vanillin’s number of oxygen atoms?
- One oxygen atom
- Two oxygen atoms
- Three oxygen atoms
- Four oxygen atoms
Correct Answer: Three oxygen atoms
Q43. In structure-activity studies, modification of vanillin’s phenolic OH often alters which property?
- Optical rotation only
- Flavor profile and antioxidant activity
- Atomic weight
- Number of carbons
Correct Answer: Flavor profile and antioxidant activity
Q44. Which laboratory reagent would selectively methylate the phenolic OH in vanillin to form methylated derivatives?
- Sodium borohydride (NaBH4)
- Diazomethane
- PCC (pyridinium chlorochromate)
- Sodium hydroxide only
Correct Answer: Diazomethane
Q45. Which safety statement about vanillin is most appropriate for pharmacists?
- Vanillin is non-toxic at any dose and requires no handling precautions
- Vanillin is an explosive compound and must be stored under inert atmosphere
- Vanillin is generally safe but may cause irritation or allergic reaction in sensitive individuals
- Vanillin is a Schedule I controlled drug
Correct Answer: Vanillin is generally safe but may cause irritation or allergic reaction in sensitive individuals
Q46. Which transformation converts vanillin into an ether derivative by replacing the phenolic hydrogen?
- Etherification (alkylation) of the phenolic OH
- Hydrogenation of aldehyde
- Oxidative cleavage of aromatic ring
- Formation of a hydrazone
Correct Answer: Etherification (alkylation) of the phenolic OH
Q47. Which solvent system is most appropriate for extracting vanillin from aqueous reaction mixtures?
- n-Hexane
- Ethyl acetate
- Water only
- Liquid ammonia
Correct Answer: Ethyl acetate
Q48. Which is a common pharmacological study area where vanillin is used as a model compound?
- Anticoagulant mechanism studies
- Permeation enhancement and drug delivery flavor masking
- Insulin replacement research
- Radioactive tracer synthesis
Correct Answer: Permeation enhancement and drug delivery flavor masking
Q49. Which of the following statements about vanillin stability is correct?
- Vanillin is highly stable to strong oxidizing agents
- Vanillin is stable under all pH conditions without degradation
- Vanillin is sensitive to strong oxidizing conditions and prolonged heating
- Vanillin cannot be oxidized under laboratory conditions
Correct Answer: Vanillin is sensitive to strong oxidizing conditions and prolonged heating
Q50. Which application area beyond flavor and fragrance increasingly explores vanillin derivatives?
- Semiconductor manufacturing
- Pharmaceutical intermediates and medicinal chemistry
- Asphalt production
- Explosive manufacturing
Correct Answer: Pharmaceutical intermediates and medicinal chemistry

I am a Registered Pharmacist under the Pharmacy Act, 1948, and the founder of PharmacyFreak.com. I hold a Bachelor of Pharmacy degree from Rungta College of Pharmaceutical Science and Research. With a strong academic foundation and practical knowledge, I am committed to providing accurate, easy-to-understand content to support pharmacy students and professionals. My aim is to make complex pharmaceutical concepts accessible and useful for real-world application.
Mail- Sachin@pharmacyfreak.com