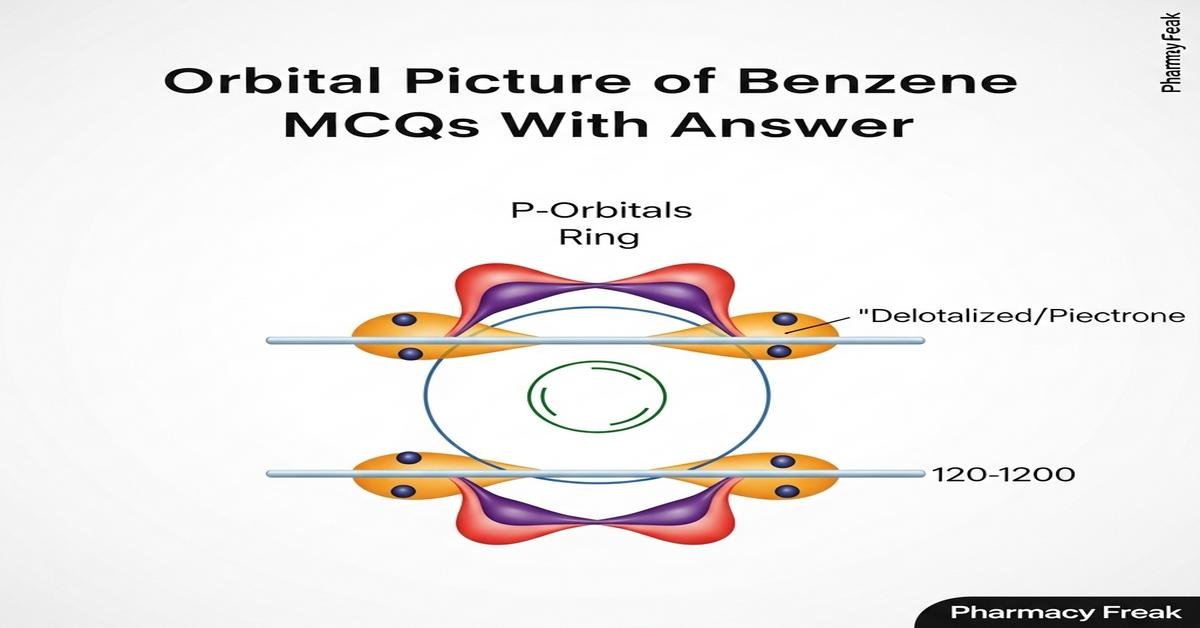
Orbital picture of benzene MCQs With Answer provides B.Pharm students a focused exploration of benzene’s molecular orbital framework, aromatic stabilization, and how delocalized 2p electrons determine reactivity and spectroscopy. This introduction covers key concepts such as sp2 hybridization, the six 2pz-derived π molecular orbitals, HOMO–LUMO features, Hückel’s 4n+2 rule, resonance energy, and implications for NMR and UV–Vis behavior. Clear, exam-relevant MCQs reinforce understanding of orbital symmetry, nodal patterns, and reaction preferences like electrophilic aromatic substitution. The set is tailored to bridge theory and pharmaceutical applications, improving problem-solving for medicinal chemistry and drug-design contexts. Now let’s test your knowledge with 50 MCQs on this topic.
Q1. Which atomic orbitals overlap to form the π system in benzene?
- Carbon 2s orbitals
- Carbon 2pz orbitals
- Hydrogen 1s orbitals
- Carbon sp2 hybrid orbitals
Correct Answer: Carbon 2pz orbitals
Q2. What is the hybridization of each carbon atom in benzene?
- sp
- sp2
- sp3
- sp3d
Correct Answer: sp2
Q3. How many π electrons are present in benzene’s delocalized system?
- 4 π electrons
- 5 π electrons
- 6 π electrons
- 8 π electrons
Correct Answer: 6 π electrons
Q4. Which rule explains benzene’s aromatic stability quantitatively?
- Baird’s rule
- Aromatic sextet rule
- Hückel’s rule (4n+2)
- Nernst rule
Correct Answer: Hückel’s rule (4n+2)
Q5. How many molecular orbitals result from combining six 2pz atomic orbitals in benzene?
- 3 molecular orbitals
- 4 molecular orbitals
- 6 molecular orbitals
- 12 molecular orbitals
Correct Answer: 6 molecular orbitals
Q6. How many doubly degenerate sets of π molecular orbitals does benzene have?
- One doubly degenerate set
- Two doubly degenerate sets
- Three doubly degenerate sets
- No degenerate sets
Correct Answer: Two doubly degenerate sets
Q7. How many electrons occupy the HOMO level(s) of benzene?
- 2 electrons
- 4 electrons
- 6 electrons
- 0 electrons
Correct Answer: 4 electrons
Q8. What is the degeneracy of benzene’s LUMO?
- Non-degenerate
- Doubly degenerate
- Triply degenerate
- Quadruply degenerate
Correct Answer: Doubly degenerate
Q9. How many nodal planes (around the ring) are present in benzene’s highest-energy π molecular orbital?
- Zero nodal planes
- One nodal plane
- Two nodal planes
- Three nodal planes
Correct Answer: Three nodal planes
Q10. Which concept best accounts for benzene’s extra stability compared with localized cyclohexatriene?
- Inductive effect
- Hyperconjugation
- Delocalization of π electrons (aromatic stabilization)
- Steric hindrance
Correct Answer: Delocalization of π electrons (aromatic stabilization)
Q11. Which resonance contributors are principal in describing benzene’s π electronic structure?
- A single Kekulé structure
- Two equivalent Kekulé structures
- Three localized double-bond structures
- Only ionic structures
Correct Answer: Two equivalent Kekulé structures
Q12. How does the diamagnetic ring current in benzene affect proton NMR chemical shifts of ring protons?
- It causes significant shielding (upfield shifts)
- It causes deshielding (downfield shifts)
- No effect on chemical shift
- It splits the signals into many peaks
Correct Answer: It causes deshielding (downfield shifts)
Q13. What is the approximate C–C bond length in benzene due to delocalization?
- 1.20 Å
- 1.34 Å
- 1.39 Å
- 1.54 Å
Correct Answer: 1.39 Å
Q14. According to Clar’s sextet theory, how many π sextets does benzene contain?
- Zero sextets
- One sextet
- Two sextets
- Three sextets
Correct Answer: One sextet
Q15. Compared to Kekulé structures, what does the molecular orbital picture emphasize?
- Localized double bonds only
- Delocalized π orbitals spanning the whole ring
- Only σ-bonding interactions
- Ionic character of benzene
Correct Answer: Delocalized π orbitals spanning the whole ring
Q16. The dominant UV–Vis transition in benzene associated with π electrons is classified as:
- n→σ* transition
- σ→σ* transition
- π→π* transition
- n→π* transition
Correct Answer: π→π* transition
Q17. Ionization of benzene (removal of an electron) primarily removes an electron from which orbital?
- The lowest π orbital
- The σ C–H orbital
- The HOMO (highest occupied π orbital)
- The LUMO
Correct Answer: The HOMO (highest occupied π orbital)
Q18. When benzene is reduced by addition of an electron, which orbital is the incoming electron placed into?
- The lowest σ orbital
- The HOMO
- The LUMO (lowest unoccupied π orbital)
- A nonbonding orbital
Correct Answer: The LUMO (lowest unoccupied π orbital)
Q19. Which set of conditions is required by Hückel’s rule for aromaticity?
- Cyclic, nonplanar, conjugated, 4n electrons
- Cyclic, planar, conjugated, 4n+2 π electrons
- Acyclic, planar, conjugated, 4n+2 π electrons
- Cyclic, planar, saturated, 4n+2 electrons
Correct Answer: Cyclic, planar, conjugated, 4n+2 π electrons
Q20. Which substituent increases electron density on the benzene ring through resonance (+R effect)?
- Nitro (−NO2)
- Fluoro (−F)
- Hydroxyl (−OH)
- Trifluoromethyl (−CF3)
Correct Answer: Hydroxyl (−OH)
Q21. The lowest-energy π molecular orbital in benzene has how many nodal planes around the ring?
- Zero nodal planes
- One nodal plane
- Two nodal planes
- Three nodal planes
Correct Answer: Zero nodal planes
Q22. Which simple theoretical method is widely used to illustrate benzene π molecular orbitals qualitatively?
- Hartree–Fock only
- Hückel molecular orbital (HMO) method
- Möller–Plesset perturbation theory
- Coupled cluster theory
Correct Answer: Hückel molecular orbital (HMO) method
Q23. In the Hückel approximation, what does the parameter β represent?
- The Coulomb integral
- The resonance (interaction) integral between adjacent p orbitals
- The kinetic energy of σ electrons
- The energy of hydrogen atoms
Correct Answer: The resonance (interaction) integral between adjacent p orbitals
Q24. Why does benzene prefer electrophilic aromatic substitution (EAS) over addition reactions?
- Addition increases conjugation and stability
- Addition would disrupt aromatic stabilization
- EAS breaks aromaticity temporarily and is irreversible
- Substitution involves σ-bond cleavage exclusively
Correct Answer: Addition would disrupt aromatic stabilization
Q25. Which species is aromatic and isoelectronic in π count with benzene (6 π electrons)?
- Cyclobutadiene
- Cyclopentadienyl anion
- Butadiene
- Cyclohexadienyl cation with 5 π electrons
Correct Answer: Cyclopentadienyl anion
Q26. Which cyclic conjugated system is classically antiaromatic due to having 4 π electrons?
- Cyclopropenyl cation
- Cyclobutadiene
- Benzene
- Cyclopentadienyl anion
Correct Answer: Cyclobutadiene
Q27. In a π molecular orbital diagram, what does a node between adjacent p orbitals indicate?
- In-phase overlap
- Out-of-phase overlap (phase change)
- σ-bonding interaction
- Hydrogen bonding
Correct Answer: Out-of-phase overlap (phase change)
Q28. Where is π electron density primarily located in benzene?
- In the plane of the ring on carbon atoms
- Above and below the ring plane
- Only at the C–C bond centers in the plane
- On the hydrogen atoms
Correct Answer: Above and below the ring plane
Q29. Are benzene π molecular orbitals localized on individual C=C bonds or delocalized over the whole ring?
- Localized on alternating C=C bonds only
- Delocalized over all six carbon atoms
- Localized on hydrogen atoms
- Localized between nonadjacent carbons exclusively
Correct Answer: Delocalized over all six carbon atoms
Q30. Photoelectron spectroscopy (PES) of benzene typically shows how many major ionization peaks for π electrons corresponding to occupied π levels?
- One peak
- Two peaks
- Three peaks
- Six peaks
Correct Answer: Three peaks
Q31. A relatively large HOMO–LUMO energy gap in benzene contributes to which property?
- High chemical reactivity toward addition
- Low chemical stability
- Thermodynamic stability and low tendency for π→π* excitations at long wavelengths
- Strong radical character
Correct Answer: Thermodynamic stability and low tendency for π→π* excitations at long wavelengths
Q32. Which reaction type is most commonly observed for benzene under normal conditions?
- Radical addition
- Electrophilic aromatic substitution (EAS)
- Nucleophilic addition to the ring π system
- Pericyclic cycloaddition on the ring
Correct Answer: Electrophilic aromatic substitution (EAS)
Q33. Aromatic stabilization arises primarily from which orbital interaction?
- Overlap of sp3 orbitals around the ring
- Continuous overlap of adjacent 2pz orbitals forming a cyclic π system
- Hydrogen bonding between ring hydrogens
- Dipole–dipole interactions only
Correct Answer: Continuous overlap of adjacent 2pz orbitals forming a cyclic π system
Q34. How many σ bonds are present in benzene (counting C–C and C–H σ bonds)?
- 6 σ bonds
- 9 σ bonds
- 12 σ bonds
- 18 σ bonds
Correct Answer: 12 σ bonds
Q35. In the benzene radical cation, how is the unpaired electron best described?
- Localized on a single carbon atom
- Delocalized over the π system
- Located on a hydrogen atom
- Confined to the σ framework
Correct Answer: Delocalized over the π system
Q36. Are the π electrons in benzene localized in alternating double bonds in the ground state?
- Yes, they are fully localized
- No, they are delocalized across the ring
- They are localized on hydrogens
- They are only present in σ orbitals
Correct Answer: No, they are delocalized across the ring
Q37. Which spectroscopic technique directly probes molecular orbital ionization energies experimentally?
- Infrared (IR) spectroscopy
- Ultraviolet–visible (UV–Vis) spectroscopy
- Photoelectron spectroscopy (PES)
- Nuclear magnetic resonance (NMR)
Correct Answer: Photoelectron spectroscopy (PES)
Q38. The resonance (stabilization) energy of benzene relative to hypothetical cyclohexatriene is approximately:
- 5 kcal/mol
- 20 kcal/mol
- 36 kcal/mol
- 100 kcal/mol
Correct Answer: 36 kcal/mol
Q39. Bonding between adjacent p orbitals in benzene requires which phase relationship?
- Out-of-phase overlap only
- In-phase overlap (same sign lobes overlapping)
- No overlap at all
- Overlap through the hydrogen atoms
Correct Answer: In-phase overlap (same sign lobes overlapping)
Q40. How many formal π bonds are counted in benzene when using simple Lewis structures?
- 1 π bond
- 2 π bonds
- 3 π bonds
- 6 π bonds
Correct Answer: 3 π bonds
Q41. Are the σ-framework and π-system in benzene orthogonal to each other?
- No, they are colinear
- Yes, σ bonds lie in the ring plane while π orbitals are perpendicular
- They are both perpendicular to the ring plane
- They overlap strongly causing σ–π mixing
Correct Answer: Yes, σ bonds lie in the ring plane while π orbitals are perpendicular
Q42. Electron-donating substituents typically direct electrophilic aromatic substitution to which positions?
- Meta only
- Ortho and para positions
- Only para position
- Anti positions
Correct Answer: Ortho and para positions
Q43. According to Frontier Molecular Orbital (FMO) theory, which orbitals interact principally during electrophilic aromatic substitution?
- Nucleophile LUMO with electrophile HOMO
- Aromatic HOMO with electrophile LUMO
- Both HOMOs only
- σ orbitals of benzene only
Correct Answer: Aromatic HOMO with electrophile LUMO
Q44. Benzene’s response to an external magnetic field reveals a ring current; this behavior indicates what magnetic property?
- Paramagnetism
- Diamagnetism associated with aromatic ring currents
- Ferromagnetism
- Antiferromagnetism
Correct Answer: Diamagnetism associated with aromatic ring currents
Q45. How many occupied π molecular orbitals are present in ground-state benzene?
- One occupied π orbital
- Two occupied π orbitals
- Three occupied π orbitals
- Six occupied π orbitals
Correct Answer: Three occupied π orbitals
Q46. Which of the following best describes benzene’s highest occupied π MOs in terms of degeneracy?
- They are non-degenerate
- They form a doubly degenerate pair
- They form a triply degenerate set
- They are all singly degenerate with different energies
Correct Answer: They form a doubly degenerate pair
Q47. The highest-energy π molecular orbital of benzene displays what pattern of phases around the ring?
- All lobes in-phase
- Alternating in-phase and out-of-phase creating three sign changes
- No sign changes around the ring
- Random phase distribution with no nodes
Correct Answer: Alternating in-phase and out-of-phase creating three sign changes
Q48. Which computational approach is commonly used for accurate visualization and quantitative energies of benzene molecular orbitals?
- Classical mechanics
- Density functional theory (DFT)
- Empirical boiling point estimation
- Simple ball-and-stick modeling
Correct Answer: Density functional theory (DFT)
Q49. The HOMO of benzene is best described as which of the following in terms of degeneracy?
- Non-degenerate and singly occupied
- Doubly degenerate and fully occupied
- Triply degenerate and unoccupied
- Non-degenerate and unoccupied
Correct Answer: Doubly degenerate and fully occupied
Q50. How many equivalent Kekulé resonance structures are required to represent benzene’s delocalization in simple valence-bond terms?
- One
- Two
- Three
- Six
Correct Answer: Two

I am a Registered Pharmacist under the Pharmacy Act, 1948, and the founder of PharmacyFreak.com. I hold a Bachelor of Pharmacy degree from Rungta College of Pharmaceutical Science and Research. With a strong academic foundation and practical knowledge, I am committed to providing accurate, easy-to-understand content to support pharmacy students and professionals. My aim is to make complex pharmaceutical concepts accessible and useful for real-world application.
Mail- Sachin@pharmacyfreak.com