Table of Contents
Introduction
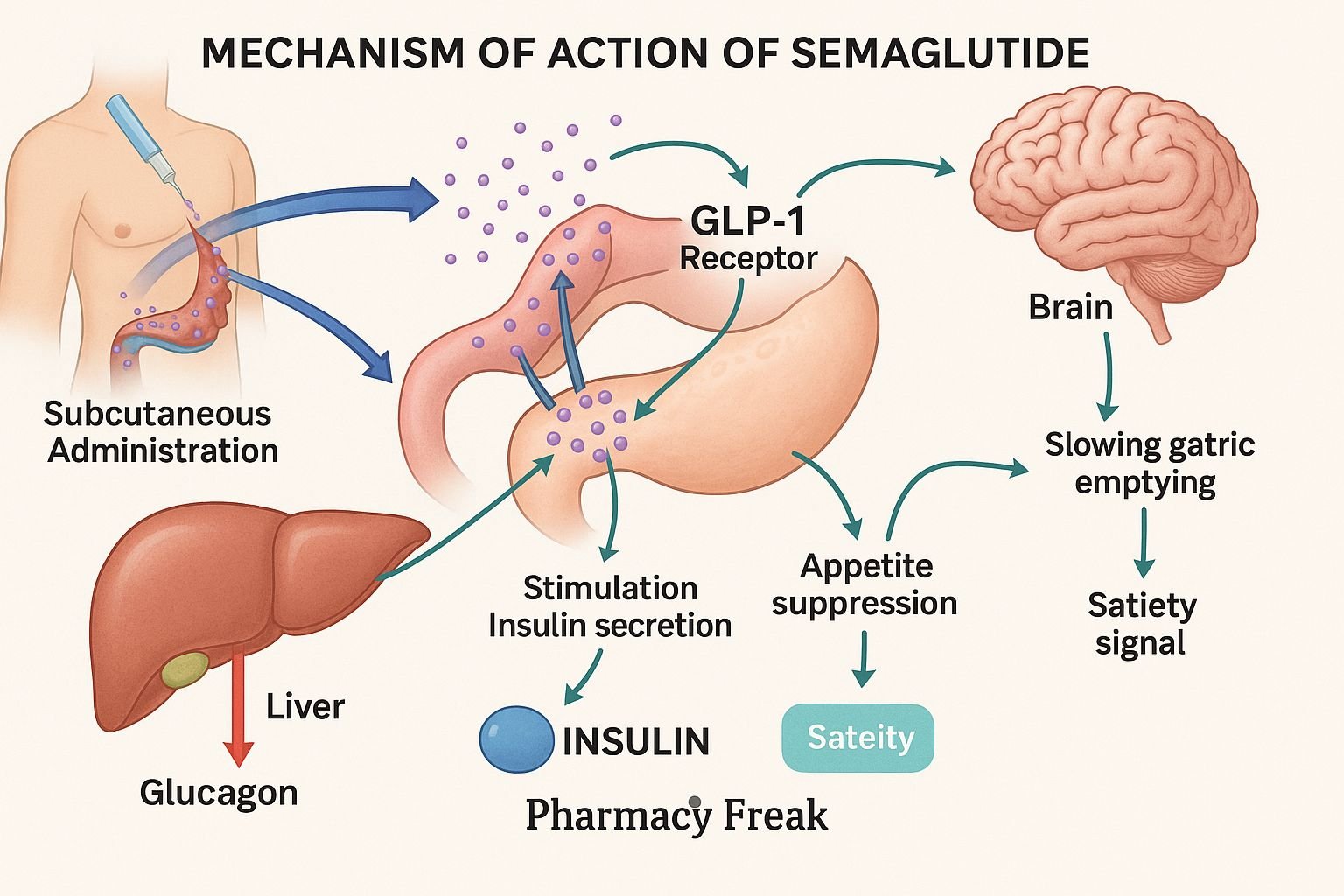
Semaglutide is a long-acting glucagon-like peptide-1 receptor agonist (GLP-1 RA) used for type 2 diabetes mellitus (T2DM) management and chronic weight management. It mimics endogenous GLP‑1 to improve glycemic control and promote weight loss through central appetite suppression and delayed gastric emptying.
Step-by-Step Mechanism of Action
- Activation of GLP‑1 receptors
Semaglutide binds to GLP-1 receptors on pancreatic β-cells, α-cells, brain satiety centers, and gastrointestinal tissue. - Glucose-dependent insulin secretion
It enhances insulin release from β-cells when blood glucose is elevated. - Suppressed glucagon secretion
By binding receptors on α-cells, it inhibits glucagon release during hyperglycemia. - Delayed gastric emptying
Slows gastric motility, reduces postprandial glucose spikes, and promotes early satiety. - Appetite suppression and weight loss
Activates satiety centers in the hypothalamus, leading to reduced caloric intake and weight loss. - Cardiovascular and renal benefits
Improves endothelial function, reduces inflammation, and decreases albuminuria—contributing to cardiovascular and renal protection.
Pharmacokinetic Parameters
| Parameter | Value |
|---|---|
| Route | Subcutaneous weekly injection |
| Bioavailability | ~89% |
| Time to Peak (Tmax) | 1–3 days |
| Protein Binding | >94% |
| Metabolism | Proteolysis; minimal CYP involvement |
| Half-life | ~165–184 hours (7 days) |
| Excretion | Metabolites excreted in urine and feces |
Clinical Uses
- Glycemic control in type 2 diabetes
- Chronic weight management in obesity and overweight (with risk comorbidities)
Adverse Effects
- Gastrointestinal: nausea, vomiting, diarrhea, constipation
- Risk of pancreatitis (rare)
- Possible gallbladder disease (e.g., cholelithiasis)
- Mild injection-site reactions
- Contraindicated in individuals with personal or family history of medullary thyroid carcinoma or MEN2
Comparative Analysis
| Agent | Receptor Duration | Dosing Interval | Weight Effect |
|---|---|---|---|
| Semaglutide | Long-acting | Weekly injection | Significant weight loss |
| Liraglutide | Short-acting | Daily injection | Moderate weight loss |
| Dulaglutide | Long-acting | Weekly injection | Moderate weight loss |
MCQs
- Semaglutide activates which receptor?
a) DPP‑4 b) GLP‑1 c) GIP d) SGLT2
Answer: b) GLP‑1 - Primary effect on β-cells is:
a) Glucose-independent insulin release
b) Glucose-dependent insulin release
c) Increased glucagon release
d) Reduced insulin synthesis
Answer: b) Glucose-dependent insulin release - It delays:
a) Gastric emptying
b) Bile secretion
c) Renal filtration
d) Insulin action
Answer: a) Gastric emptying - A central effect of semaglutide is:
a) Increased appetite
b) Appetite suppression
c) Blood pressure increase
d) Cognitive enhancement
Answer: b) Appetite suppression - The half-life allows dosing:
a) Daily b) Weekly c) Monthly d) Daily BID
Answer: b) Weekly - A potential rare adverse effect is:
a) Hypoglycemia b) Pancreatitis c) Hepatorenal syndrome d) Constipation
Answer: b) Pancreatitis - It is contraindicated in patients with:
a) Type 1 diabetes b) Medullary thyroid carcinoma c) Hyperthyroidism d) Hypothyroidism
Answer: b) Medullary thyroid carcinoma - Protein binding is approximately:
a) 50% b) 75% c) >94% d) 100%
Answer: c) >94% - Effects on heart and kidneys include:
a) Increased albuminuria b) Vascular protection c) Fluid retention d) Increased LDL
Answer: b) Vascular protection - Compared to liraglutide, semaglutide dosing is:
a) Daily b) Weekly c) Monthly d) BID
Answer: b) Weekly
FAQs
1. How often is semaglutide administered?
It’s given once weekly via subcutaneous injection.
2. Does it cause hypoglycemia?
Hypoglycemia risk is low when used alone but increases if combined with insulin or sulfonylureas.
3. How much weight loss can be expected?
On average, patients lose 5–15% of body weight over several months.
4. Should thyroid function be monitored?
Side effects on thyroid are rare but avoid in those with medullary thyroid carcinoma or MEN2.
5. Is it safe in patients with renal impairment?
Semaglutide requires no dose adjustment in mild-to-moderate renal impairment.
References
- Goodman & Gilman’s The Pharmacological Basis of Therapeutics
- KD Tripathi. Essentials of Medical Pharmacology
- DrugBank: Semaglutide pharmacology summary
- StatPearls: GLP-1 receptor agonists
- PubMed review: Semaglutide cardiovascular and weight benefits
I am pursuing MBA in pharmaceutical management from NIPER Hyderabad with a strong academic record and proven success in national-level pharmacy entrance exams. I secured AIR 61 in NIPER 2024 (MS/M.Pharm) and AIR 27 in NIPER MBA, along with AIR 147 in GPAT 2024 and AIR 907 in GPAT 2023. I also achieved AIR 6 in AIIMS CRE-2025 for Drug Store Keeper and was selected as a Pharmacist (AIR 61) for ESIC. Additionally, I was the Runner-Up in Round 2 of the EY Case Study Competition.
At PharmacyFreak.com, I aim to guide future pharmacists through expert content, exam strategies, and insightful resources based on real experience and academic excellence.
Mail- harsh@pharmacyfreak.com