Introduction
This quiz collection focuses on linear and non-linear compartment models in pharmacokinetics, tailored for M.Pharm students preparing for Advanced Pharmacology-I. It explains core concepts such as first-order and zero-order kinetics, Michaelis–Menten elimination, capacity-limited clearance, and target-mediated drug disposition (TMDD). Questions emphasize model selection, parameter interpretation (Vmax, Km, clearance, volume), dose-proportionality, identifiability, and implications for dosing and therapeutic monitoring. The set mixes theoretical, mathematical and applied scenarios to deepen understanding of when linear assumptions break down and how to analyze and interpret non-linear behavior using compartmental modelling and population approaches. Use these MCQs to test and reinforce advanced PK reasoning and problem-solving.
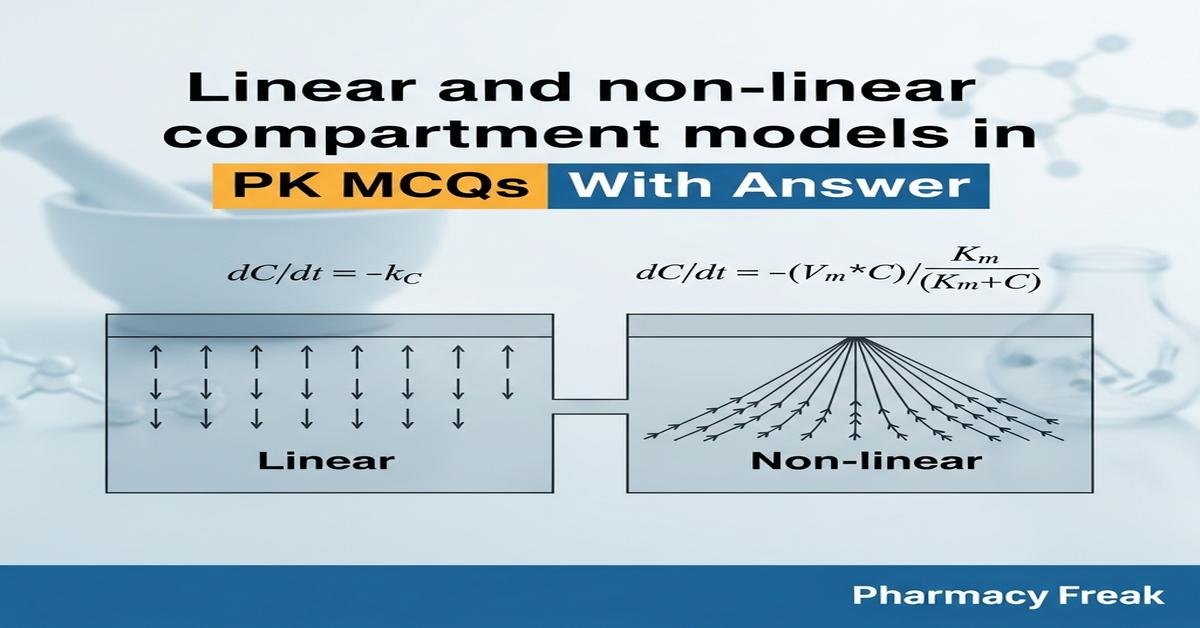
Q1. What is the primary distinction between a linear and a non-linear pharmacokinetic model?
- Linear models have parameters independent of drug concentration; non-linear models have parameters that change with concentration.
- Linear models use compartments; non-linear models do not use compartments.
- Linear models always assume zero-order absorption; non-linear models assume first-order absorption.
- Linear models apply only to intravenous dosing; non-linear models apply only to oral dosing.
Correct Answer: Linear models have parameters independent of drug concentration; non-linear models have parameters that change with concentration.
Q2. Michaelis–Menten elimination is best described by which rate equation for elimination rate (v)?
- v = k · C
- v = Vmax · C / (Km + C)
- v = Vmax · e^(−Km·C)
- v = CL · C
Correct Answer: v = Vmax · C / (Km + C)
Q3. In a Michaelis–Menten elimination process, what happens to clearance (CL = v/C) as concentration C increases well above Km?
- Clearance approaches Vmax/Km and becomes constant.
- Clearance increases linearly with concentration.
- Clearance decreases toward zero and becomes negligible.
- Clearance approaches Vmax/C and therefore decreases with increasing C.
Correct Answer: Clearance approaches Vmax/C and therefore decreases with increasing C.
Q4. Which of the following is a characteristic clinical implication of non-linear (saturable) elimination?
- Small dose increases may produce disproportionately large increases in drug exposure (AUC).
- Drug exposure (AUC) doubles when dose doubles.
- Half-life remains constant across all dose ranges.
- Clearance is independent of concentration.
Correct Answer: Small dose increases may produce disproportionately large increases in drug exposure (AUC).
Q5. Which graphical method is commonly used to linearize Michaelis–Menten kinetics for parameter estimation?
- Lineweaver–Burk plot (1/v vs 1/C).
- Henderson–Hasselbalch plot (pH vs log ratio).
- Kaplan–Meier survival curve.
- Arrhenius plot (ln k vs 1/T).
Correct Answer: Lineweaver–Burk plot (1/v vs 1/C).
Q6. In a one-compartment linear model after an IV bolus, concentration-time follows which mathematical form?
- Exponential decline: C = C0 · e^(−kel·t).
- Power law decline: C = C0 · t^(−α).
- Linear decline: C = C0 − k·t.
- Sigmoidal rise then decline: C = Cmax/(1+e^(−k(t−t0))).
Correct Answer: Exponential decline: C = C0 · e^(−kel·t).
Q7. Which parameter set uniquely defines linear two-compartment IV bolus model behaviour?
- Volume of central compartment (Vc), intercompartmental clearance (Q), peripheral volume (Vp), and systemic clearance (CL).
- Vmax, Km, absorption rate constant (ka), and bioavailability (F).
- Only half-life and Cmax.
- Elimination rate constant and Michaelis constant only.
Correct Answer: Volume of central compartment (Vc), intercompartmental clearance (Q), peripheral volume (Vp), and systemic clearance (CL).
Q8. Target-mediated drug disposition (TMDD) typically results in non-linear PK because:
- Drug binds to a limited number of high-affinity receptors, causing saturable elimination or distribution.
- Drug is metabolized only by abundant non-saturable enzymes.
- Drug absorption is always zero-order.
- Drug excretion occurs only via unchanged renal filtration.
Correct Answer: Drug binds to a limited number of high-affinity receptors, causing saturable elimination or distribution.
Q9. For a drug with Km much larger than therapeutic concentrations, which approximation is valid for elimination?
- First-order (linear) elimination with rate ≈ (Vmax/Km)·C.
- Zero-order elimination independent of concentration.
- Elimination follows sigmoidal Emax kinetics.
- Elimination is proportional to C^2.
Correct Answer: First-order (linear) elimination with rate ≈ (Vmax/Km)·C.
Q10. Which statement best describes dose-proportionality in linear pharmacokinetics?
- AUC increases proportionally with dose; Cmax increases proportionally with dose.
- AUC increases more than proportionally with dose while Cmax stays constant.
- AUC is independent of dose.
- AUC decreases as dose increases due to saturation.
Correct Answer: AUC increases proportionally with dose; Cmax increases proportionally with dose.
Q11. Which parameter is directly estimated from Michaelis–Menten kinetics and represents the concentration at half-maximal elimination rate?
- Km
- Vmax
- CL
- Vc
Correct Answer: Km
Q12. In non-linear mixed-effects (population PK) modelling of saturable kinetics, which approach is most appropriate for estimating Vmax and Km from sparse clinical data?
- Use a population model (e.g., NONMEM) fitting a Michaelis–Menten structural model with between-subject variability.
- Use simple linear regression of AUC vs dose.
- Assume linear PK and estimate CL only.
- Use Kaplan–Meier analysis to estimate parameters.
Correct Answer: Use a population model (e.g., NONMEM) fitting a Michaelis–Menten structural model with between-subject variability.
Q13. Which behavior of half-life indicates non-linear elimination?
- Half-life increases with increasing concentration or dose.
- Half-life remains constant over all doses.
- Half-life decreases linearly with time.
- Half-life equals the dosing interval always.
Correct Answer: Half-life increases with increasing concentration or dose.
Q14. Which scenario can produce apparent non-linearity in PK even if elimination is linear?
- Saturable absorption or saturable plasma protein binding at therapeutic concentrations.
- Infusion at constant rate into a linear one-compartment model.
- Perfect linear superposition of independent dosing events.
- Complete first-pass hepatic metabolism unaffected by concentration.
Correct Answer: Saturable absorption or saturable plasma protein binding at therapeutic concentrations.
Q15. When fitting a two-compartment non-linear model, identifiability problems typically arise due to:
- Sparse sampling in the distribution phase and correlation among parameters leading to non-unique solutions.
- Excessive data across many doses making the model overdetermined.
- Linear elimination making parameters trivial to estimate.
- Use of terminal half-life only to estimate all compartments.
Correct Answer: Sparse sampling in the distribution phase and correlation among parameters leading to non-unique solutions.
Q16. For a drug eliminated by saturable hepatic metabolism with Vmax = 500 mg/h and Km = 10 mg/L, what happens to elimination rate as concentration rises from 1 mg/L to 100 mg/L?
- The elimination rate approaches Vmax and thus increases less proportionally at high concentration.
- The elimination rate remains strictly proportional to concentration across this range.
- The elimination rate decreases to zero as concentration increases.
- The elimination rate becomes negative at high concentrations.
Correct Answer: The elimination rate approaches Vmax and thus increases less proportionally at high concentration.
Q17. Which measure indicates accumulation at steady state for linear PK with dosing interval τ and elimination rate constant kel?
- Accumulation ratio R = 1 / (1 − e^(−kel·τ)).
- R = Vmax / Km.
- R = CL / Vc.
- R = ka / kel.
Correct Answer: Accumulation ratio R = 1 / (1 − e^(−kel·τ)).
Q18. In a non-linear PK system showing Michaelis–Menten elimination, which dosing adjustment is most prudent when approaching saturation?
- Reduce incremental dose increases and monitor plasma concentrations closely to avoid disproportionate exposure.
- Increase dose substantially to overcome saturation quickly.
- Assume linearity and continue standard proportional dosing.
- Discontinue all dose adjustments; saturation has no clinical impact.
Correct Answer: Reduce incremental dose increases and monitor plasma concentrations closely to avoid disproportionate exposure.
Q19. Which statement about linear superposition in compartmental models is true?
- Linear superposition applies only to linear systems and allows prediction of multiple-dose profiles by summing single-dose responses.
- Linear superposition is valid for any non-linear system including Michaelis–Menten elimination.
- Linear superposition requires zero-order elimination exclusively.
- Linear superposition predicts accumulation only for drugs with saturable kinetics.
Correct Answer: Linear superposition applies only to linear systems and allows prediction of multiple-dose profiles by summing single-dose responses.
Q20. Which experimental observation most strongly suggests target-mediated drug disposition (TMDD) rather than simple Michaelis–Menten metabolism?
- Concentration-dependent distribution with rapid initial nonlinear decline due to high-affinity binding to receptors and delayed terminal phase.
- Simple proportional increase in AUC with dose across all ranges.
- Single exponential decline from C0 with constant half-life at all doses.
- No effect of receptor expression or target saturation on PK.
Correct Answer: Concentration-dependent distribution with rapid initial nonlinear decline due to high-affinity binding to receptors and delayed terminal phase.

I am a Registered Pharmacist under the Pharmacy Act, 1948, and the founder of PharmacyFreak.com. I hold a Bachelor of Pharmacy degree from Rungta College of Pharmaceutical Science and Research. With a strong academic foundation and practical knowledge, I am committed to providing accurate, easy-to-understand content to support pharmacy students and professionals. My aim is to make complex pharmaceutical concepts accessible and useful for real-world application.
Mail- Sachin@pharmacyfreak.com