Instrumentation of AAS MCQs With Answer
Atomic Absorption Spectroscopy (AAS) remains a cornerstone technique in pharmaceutical elemental analysis due to its selectivity, robustness, and wide dynamic range. For M. Pharm students, mastering AAS instrumentation is essential to ensure accurate quantification of trace metals in APIs, excipients, and dosage forms. This quiz focuses on the practical and theoretical aspects of AAS hardware: radiation sources, atomizers, optical systems, detectors, background correction strategies, and sample introduction components. You’ll apply concepts on flame and graphite furnace setups, hydride generation, cold vapor techniques, and common interferences with their remedies. Each question is designed to test depth of understanding, relate to real analytical decisions, and reinforce best practices for method development and validation in modern pharmaceutical laboratories.
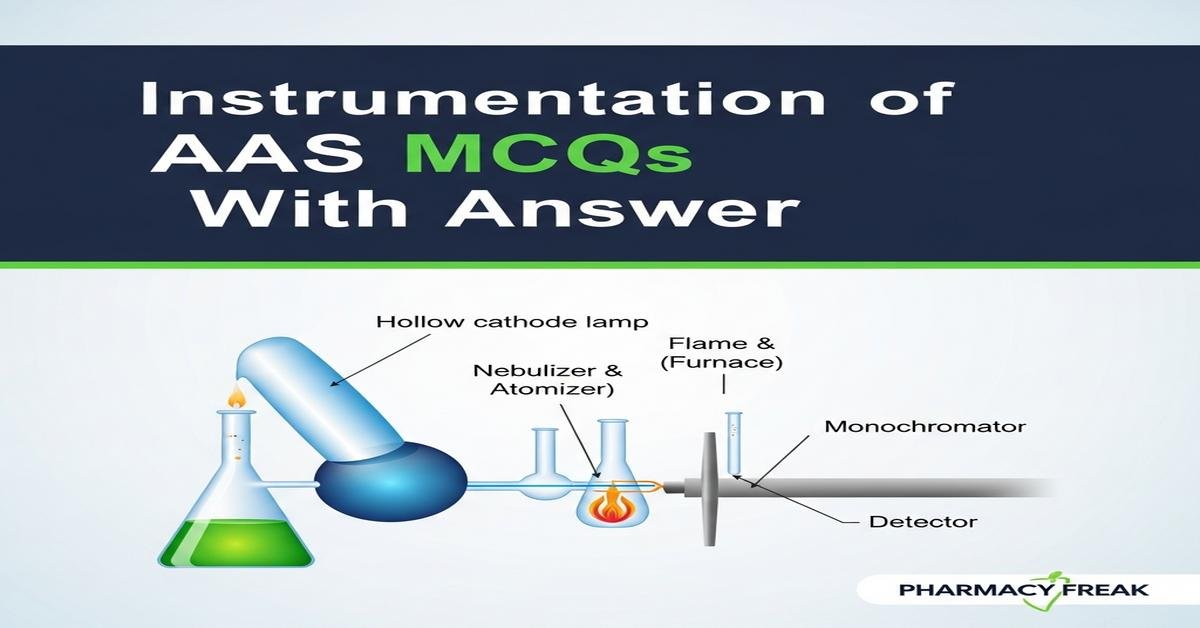
Q1. In line-source AAS, the primary radiation source matched to the analyte element is which?
- Tungsten-halogen continuum lamp
- Deuterium arc lamp
- Hollow cathode lamp containing the analyte element
- Xenon flash lamp
Correct Answer: Hollow cathode lamp containing the analyte element
Q2. The main function of the nebulizer–spray chamber in flame AAS is to:
- Atomize the sample to free atoms
- Convert the liquid sample into a fine aerosol and deliver a stable fraction to the burner
- Ionize atoms to increase sensitivity
- Separate isotopes before detection
Correct Answer: Convert the liquid sample into a fine aerosol and deliver a stable fraction to the burner
Q3. For refractory elements requiring higher atomization temperature, the preferred flame–oxidant combination is:
- Air–acetylene (lean)
- Nitrous oxide–acetylene
- Hydrogen–air
- Propane–air
Correct Answer: Nitrous oxide–acetylene
Q4. Compared with flame AAS, a key instrumental advantage of graphite furnace AAS is:
- Ability to measure nonmetals via emission
- Elimination of background absorption
- Higher atom residence time leading to lower detection limits for microliter samples
- No need for calibration standards
Correct Answer: Higher atom residence time leading to lower detection limits for microliter samples
Q5. The monochromator in AAS is primarily used to:
- Increase lamp intensity
- Isolate the analyte resonance line and reject stray light
- Align the lamp to the burner head
- Cool the detector to reduce noise
Correct Answer: Isolate the analyte resonance line and reject stray light
Q6. Which detector is most commonly used in traditional line-source AAS in the UV–Vis region?
- Photomultiplier tube
- Thermocouple
- Flame ionization detector
- Bolometer
Correct Answer: Photomultiplier tube
Q7. The purpose of a beam chopper or rotating mirror in a double-beam AAS is to:
- Increase flame temperature
- Alternate the radiation between sample and reference paths to correct for source fluctuations and flame emission
- Focus the beam onto the entrance slit
- Split the beam into multiple analytical lines
Correct Answer: Alternate the radiation between sample and reference paths to correct for source fluctuations and flame emission
Q8. Deuterium lamp background correction operates by:
- Applying a magnetic field to split absorption lines
- Measuring molecular absorption/scattering using a continuum source and subtracting it from total absorption
- Modulating lamp current to periodically broaden the emission line
- Using a second monochromator to isolate the background
Correct Answer: Measuring molecular absorption/scattering using a continuum source and subtracting it from total absorption
Q9. Zeeman background correction in electrothermal AAS is based on:
- High-frequency modulation of the hollow cathode lamp
- A rotating grating scanning across the line
- Application of a magnetic field to split the atomic line and differentiate analyte absorption from background
- Use of an additional deuterium lamp
Correct Answer: Application of a magnetic field to split the atomic line and differentiate analyte absorption from background
Q10. Hydride generation AAS is especially suited for determination of:
- Alkali metals (Na, K)
- Hydride-forming elements such as As, Se, Sb, Bi
- Halogens (Cl, Br)
- Rare earth elements
Correct Answer: Hydride-forming elements such as As, Se, Sb, Bi
Q11. Cold vapor AAS is primarily used for the sensitive determination of:
- Lead
- Mercury
- Zinc
- Copper
Correct Answer: Mercury
Q12. Increasing the slit width in the monochromator generally:
- Decreases signal intensity but improves resolution
- Increases signal intensity but degrades spectral resolution and may increase background
- Has no effect on spectral bandwidth
- Eliminates the need for background correction
Correct Answer: Increases signal intensity but degrades spectral resolution and may increase background
Q13. Excessive hollow cathode lamp current most commonly causes:
- Enhanced linearity over a wider range
- Self-absorption and line broadening leading to nonlinearity
- Decreased shot noise
- Elimination of stray light
Correct Answer: Self-absorption and line broadening leading to nonlinearity
Q14. In graphite furnace AAS, the correct sequence of the temperature program is:
- Atomization → Drying → Pyrolysis → Clean-out
- Drying → Pyrolysis → Atomization → Clean-out
- Drying → Atomization → Pyrolysis → Clean-out
- Pyrolysis → Drying → Atomization → Clean-out
Correct Answer: Drying → Pyrolysis → Atomization → Clean-out
Q15. Matrix modifiers (e.g., Pd/Mg(NO3)2) are added in electrothermal AAS primarily to:
- Increase lamp intensity
- Stabilize the analyte during pyrolysis so higher char temperatures can be used, minimizing matrix interferences
- Reduce the sample volume required
- Cool the graphite tube to extend its life
Correct Answer: Stabilize the analyte during pyrolysis so higher char temperatures can be used, minimizing matrix interferences
Q16. The main role of the slot burner head in flame AAS is to:
- Shorten the optical path to prevent self-absorption
- Provide a long, stable optical path and minimize flame flicker noise
- Intensify turbulence to mix gases
- Focus radiation into the monochromator
Correct Answer: Provide a long, stable optical path and minimize flame flicker noise
Q17. Which calibration strategy is most appropriate when significant matrix effects are present in AAS?
- External calibration with water blanks
- Internal standardization using a non-absorbing element
- Standard addition directly into the sample matrix
- One-point calibration at the midrange standard
Correct Answer: Standard addition directly into the sample matrix
Q18. A releasing agent (e.g., LaCl3 in Ca determination) is used in flame AAS to:
- Increase droplet size in the aerosol
- Preferentially bind the interferent and prevent formation of refractory compounds with the analyte
- Prevent ionization by supplying excess electrons
- Cool the flame to reduce noise
Correct Answer: Preferentially bind the interferent and prevent formation of refractory compounds with the analyte
Q19. Stray light entering the monochromator/detector primarily leads to:
- Overestimation of absorbance at high concentrations, extending linearity
- Underestimation of absorbance at high concentrations, causing curvature and limited linear range
- No change in calibration linearity
- A shift in analytical wavelength
Correct Answer: Underestimation of absorbance at high concentrations, causing curvature and limited linear range
Q20. Compared with traditional line-source AAS, high-resolution continuum-source (HR-CS) AAS uses:
- A hollow cathode lamp and low-resolution monochromator
- A continuum lamp (xenon) and high-resolution echelle spectrometer with array detector for direct spectral background modeling
- A laser source and photodiode detector only
- No background correction capability
Correct Answer: A continuum lamp (xenon) and high-resolution echelle spectrometer with array detector for direct spectral background modeling

I am a Registered Pharmacist under the Pharmacy Act, 1948, and the founder of PharmacyFreak.com. I hold a Bachelor of Pharmacy degree from Rungta College of Pharmaceutical Science and Research. With a strong academic foundation and practical knowledge, I am committed to providing accurate, easy-to-understand content to support pharmacy students and professionals. My aim is to make complex pharmaceutical concepts accessible and useful for real-world application.
Mail- Sachin@pharmacyfreak.com